The Future of CAR T for Solid Tumours: Antigen Discovery, Persistence, and Fitness
The development of chimeric antigen receptor (CAR) T cell therapy has been ground-breaking in the treatment of haematological cancers. As such, the successes that the therapy has brought to the clinic and beyond has driven interest and excitement in translating it to solid tumour indications.
Solid tumours are more difficult, however. Solid cancer cells present far more multivariance in the antigens that they express: as a result, finding tumour associated antigens with the right degree of specificity is a significant challenge. In addition, reaching the tumour cell population and effective action within the tumour are made more difficult by the microenvironment as well as immune evading properties of solid tumours.
Discussing the challenges, developments, and strategies for using CAR T on solid tumours, Oxford Global hosted an Immuno series discussion group titled Cell Therapies and Solid Tumour Therapy Development. Leading the discussion were Michael Zaiac, Head of Medical Affairs Oncology Region Europe at Novartis, and Paul Rennert, CEO of Aleta Biotherapeutics.
Novartis Keeping their Finger on the Pulse
Zaiac explained that Novartis has been increasingly interested in the solid tumour space for CAR T technology. This has been due to the growth in capacity and understanding of tumour genomics. Furthermore, the ability to crunch large amounts of data, in turn predicting neoantigen targets and their ideal combinations, has piqued the company’s interest.
As such, according to Zaiac, Novartis has been keeping a keen eye on the emerging technologies in this field. Zaiac hopes that Novartis capability to create CAR T cells with a quick turnaround and hence a potentially more “natural” T cell like behaviour, could be suitable as a “consistent backbone” for new and interesting CAR T therapeutics in solid Tumors .
Algorithms designed to identify targetable neoantigens are one such technology that Zaiac referenced. Furthermore, Zaiac mentioned ‘armouring technology’ that would be able to make T cells better sustained in the tumour microenvironment.
Still, Zaiac reiterated that Novartis are currently only “dipping their toes in the water,” due to the lack of major breakthroughs in the field. Novartis has not made any R&D decisions on any single technology for the time being, but the company is investing having the diagnostics in place to have access to emerging neoantigens.
Aleta Biotherapeutics: Using CAR T Engagers
Rennert said of breaking into the solid tumour space that “it takes a village to raise a child.” Although Aleta is a small biotech company, the work they do for CAR T technology is making impressive breakthroughs.
Rennert explained that Aleta debuted in CAR T with their engager platform. Aleta-001, their inaugural biologic, will be entering the clinic in 2022 with Cancer Research UK funding. Aleta-001 links CD19 extracellular domain to anti-CD20. The very small protein solves a simple problem: the fact that CAR T tends to lead to relapses due to loss or down-regulation of CD19.
The biologic coats every molecule of CD20 with CD19. “Therefore, the CAR doesn’t care, it just sees CD19,” Rennert added. Herein, it doesn’t matter if CD19 is downregulated as the cell is coated with it. “The CAR continues to kill even if the tumour is trying to escape.”
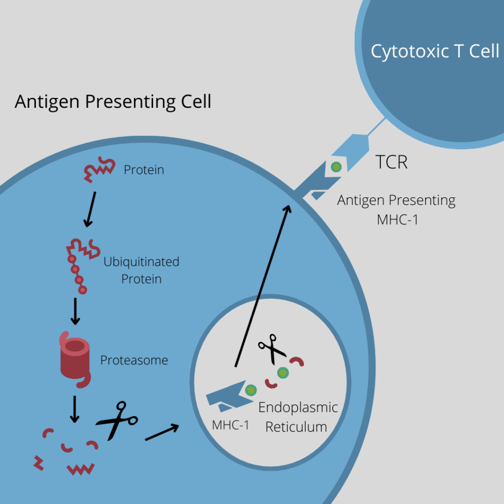
There are two benefits to this. The first is the obvious fact that the antigen density will substantially increase: “There is about a five-fold CD20 on most lymphomas compared to CD19,” said Rennert. Secondly, if the patient loses native expression of CD19, the biologic will still coat the CD20, effectively acting as a substitute for that native expression.
Aleta On Translating to Solid Tumours
Rennert then went on to discuss how Aleta is working to translate this capability into the solid tumour space. He highlighted the research he had seen which suggested that targeting solid tumours was possible with CARs. “The issue with those studies is the persistence of those CARs is limited,” said Rennert.
So, Aleta encoded a CD19–anti-HER2 CAR T engager inside their CD19 CAR, and then saw whether it was capable of attacking solid tumour cells. This meant that the engagers were encoded to be secreted extracellularly by the CAR T itself. “It works really well,” affirmed Rennert; “the potency is incredible.”
“We’re going to let those CARs wipe out the normal B cells in order to build a fit and highly persistent CAR T cell pool.”
The antigen density is so high because Aleta makes sure to always build multi-antigen targeting CAR T engagers. For example, for HER2 central nervous system (CNS) metastases, their engager targets HER2 and B7-H3 simultaneously. Further still, for paediatric CNS solid tumours, Aleta is targeting three antigens, all encoded in the same CAR T engager.
Rennert said that the problems that Aleta is currently trying to solve are countering tumour heterogeneity and improving both CAR T persistence and fitness. The prospect of using engagers for these challenges looks very promising for Rennert and the rest of Aleta.
Furthermore, the close watch of innovative Pharma companies over developments in the CAR T space (especially in neoantigen discovery, e.g., NEON and KITE neoantigen TCR technology acquired by BioNTech) seems to indicate that it is being taken seriously across the pharmaceutical industry, with plenty more developments to come.
Oxford Global’s Immuno UK: In Person conference will host talks from expert voices on designing and delivering innovative immunotherapies to transform cancer care. Gain valuable insights into the approaches impacting the immunotherapy field through 40+ outstanding presentations tackling key discussion points in immuno-oncology, immunology, and inflammation.