Mechanochemical Synthesis as a Solvent-Free Peptide Synthesis Method
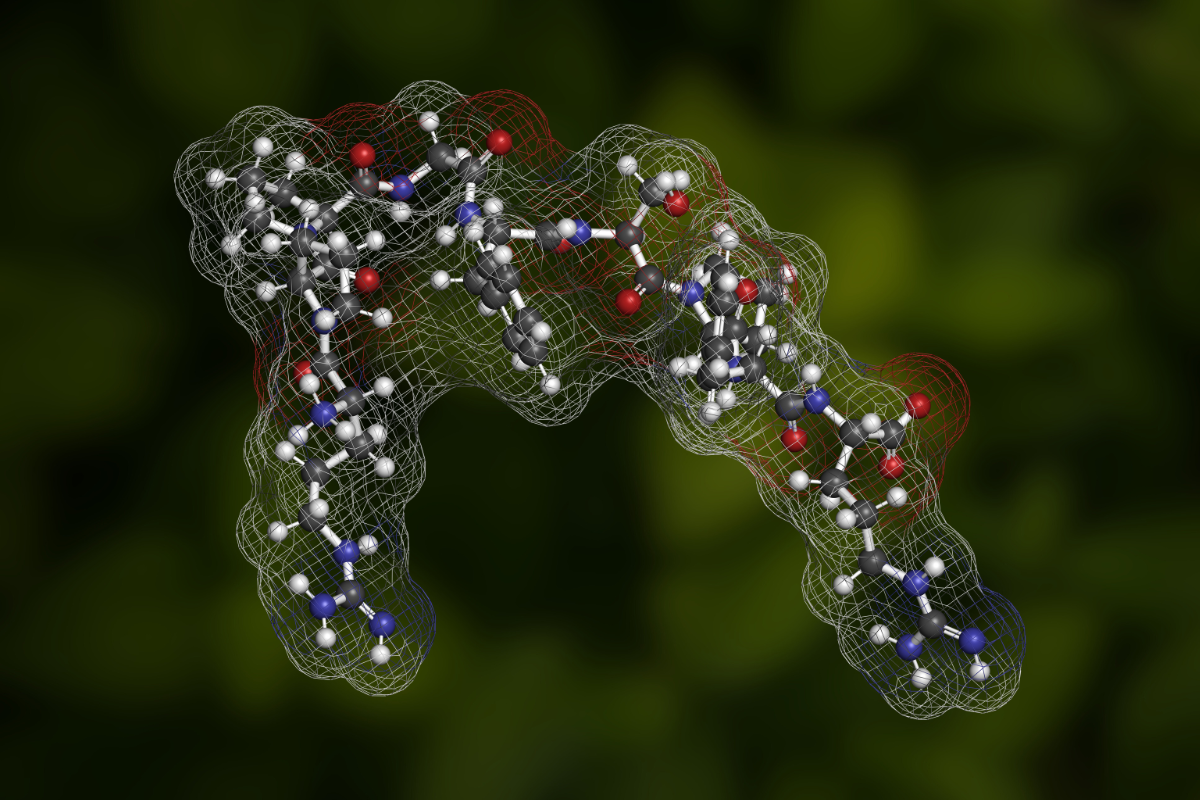
Researchers at Nagoya Institute of Technology, Japan and the Japan Science and Technology Agency (JST) have successfully developed an environmentally friendly method for the synthesis of acyl fluorides.
The study, published in the journal ACS Sustainable Chemistry & Engineering, describes their method for producing acyl fluoride from carboxylic acid without a catalyst or solvent.
RELATED:
- Sustainability in Pharmaceutical Manufacture and Healthcare Provision
- Trends and Challenges in Peptide Bioanalysis and Production
- New Peptide With Hope of Treating Triple-Negative Breast Cancer
Notably, the Nagoya team also succeeded in converting the acyl fluoride that they had synthesized into amides and peptides. The study notes that the method proposed yielded larger product amounts than conventional techniques in a shorter amount of time.
Mechanochemical synthesis
The method uses mechanochemical synthesis mediated by 1,1,2,2-tetrafluoroethyl-N,N-dimethylamine (TFEDMA) using a ball mill.
Mechanochemical synthesis is an up-and-coming method of organic synthesis that uses strong mechanical stirring to react compounds with poor solubility. Those compounds may be conventionally difficult to handle using traditional methods without the use of hazardous catalysts.
Amide bonds
The synthesis of proteins and peptides heavily relies on amide bonds. Therefore these bonds are critical to the synthesis of biopharmaceutical molecules.
Conventional methods for amide bonding are prone to side-reactions which has resulted in purity concerns. Furthermore, these coupling reactions require reagents that are prone to the production of environmentally hazardous waste products.
This is the rationalisation for the Nagoya team seeking a solution that does not require the use of solvents, catalysts, or reagents.
Green peptide synthesis
The team say that “the synthesis of acyl fluoride from carboxylic acid using the TFEDMA-mediated mechanochemical method developed in this study significantly reduces the environmental impact compared to conventional synthesis methods.”
They also say that their technology “has the potential to be strongly supported not only by industry but also by the general public as an innovative option for realising a sustainable society.”
The study was led by a research group in the Department of Chemistry, Nagoya Institute of Technology’s Graduate School of Engineering. Zhengyu Zhao (3rd year joint major in Nanomedicine Science), Sota Igawa (1st year in the Life and Applied Chemistry Program, Department of Engineering). Professor Tetsuo Shibata (joint Major in Nanomedicine Science and Major in Engineering (Life and Applied Chemistry)).Related Resources







