Harnessing Bispecific T Cell Engagers and T Cell Primers in Cancer Treatment
T cell-based bispecific antibody immunotherapies have reshaped treatment paradigms across various cancer types. The global bispecific antibody market is expected to increase to 25 billion USD by 2028, with the number of commercially available products projected to increase by 150% over the next 5 to 6 years. Harnessing the capabilities of immune cells, especially T cells, to enhance anti-tumour activity is a widely adopted strategy in the clinical management of cancer. However, challenges remain, as a wide cross-section of the population do not respond to pre-established cancer treatments, with tumours either refracting or developing resistance. There are also economic and logistical hurdles associated with scaling-up and considerable risk in systematic stimulation, which can lead to toxicity. Nevertheless, stimulating T cells can lead to transformational clinical benefits for cancer patients. Current trends in development include redirecting, reinvigorating, and activating T cells for optimal results. Key players in the field include Abbvie, Alligator Biosciences, Amgen, Bristol-Myers Squibb, F-star, Genmab, Ichnos, Janssen, Regeneron, and Roche.
Bispecific T Cell Engagers - Background and Industry Overview:
Bispecific antibodies are highly potent molecules that facilitate T cell-mediated killing of tumour cells. Simultaneously binding to tumour antigen and CD3e chains results in the activation of the T cell receptor. Subsequent T cell engagement includes the activation and killing of tumour cells by releasing cytotoxic granules. T cells start to proliferate and expand at the activation site, releasing cytokine and chemokine, which leads to the recruitment of additional T cells. Christian Klein, Site Head Roche Innovation Centre Zurich, explains how “T cell bispecifics are very potent and can mediate so-called serial killing of tumour cells”. He continued, “notably, they can be independent of specificity and the action and differentiation status of T cells”. A major advantage of T cell bispecific antibodies is that they can overcome the various escape mechanisms of cancer cells.
- Read our Discussion Group Report Addressing the Future of Bispecific and Multi-Specific Antibodies
- Check out our Commentary Article Analysing Alligator Bioscience AB and Apteov Therapeutics Inc's Joint Bispecific Antibody
“The field has now essentially moved on to IgG-based T cell bispecific antibodies, with numerous platforms in clinical trials across the industry,” Klein continued. Upcoming platforms include Amgen’s Fc-BiTE, classical 1+1 IgG based T cell bispecifics like Genentech’s TDB platform applied in Mosunetuzumab and Cevostamab, Genmab’s Duobody platform used for example in Epcoritamab with Abbvie or in Teclistamab and Talquetamb with Janssen, Regeneron’s common light chain 1+1 IgG applied to Odronextamab, and an IgG-scFv platform applied for instance by Xencor for Plamotamab with Janssen. Roche has developed its own differentiated 2+1 TCB platform applied e.g. for development of glofitamab and cibisatamab making use of bivalent binding to tumour antigens.
Peter Ellmark, Chief Scientific Officer at Alligator Bioscience concurs, explaining how, “there is a great and rapidly increasing need for ... therapies that can expand the number and quality of tumour infiltrating T cells”.
According to Klein, “the interesting aspect of the different T cell bispecific antibody formats making their way to the clinic, is that they essentially enable you to mimic CAR T cell functioning by using off the shelf reagents”. Peter Ellmark, Chief Scientific Officer at Alligator Bioscience concurs, explaining how, “there is a great and rapidly increasing need for such therapies that can expand the number and quality of tumour infiltrating T cells”.
A Promising Landscape: 3 Industry Case-Studies
From T cell priming, redirecting activated T cells, and endogenous and synthetic immunity, the possibilities are numerous. To find out more, we sat down with 3 leading pharmaceutical companies paving the way with their novel T-cell bispecific antibodies treatment strategies.
1.) Roche: ‘Roche-Genentech Cancer Immunotherapy Strategy’
Founded in 1896, Roche is a Swiss multinational healthcare leader that focuses on delivering life-saving pharmaceuticals and cutting-edge diagnostics. Roche-Genentech’s strategy relies on a multi-functional approach to cancer immunotherapy, which makes use of endogenous immunity approaches, that rely on pre-existing T cells that have been formed by the body’s immune system to attack the cancer for example by blocking the PD-L1 checkpoint inhibitor or other notable checkpoint inhibitors including TIGIT (tiragolumab), or by simultaneously blocking PD-1 and LAG-3 with a bispecific antibody.
In addition, the synthetic immunity approach is a direction in which Roche and Genentech have various T cell engaging bispecific antibodies in development such as cibisatamab, glofitamab, mosunetuzumab or cevostamab. Roche’s portfolio also harnesses targeted immunomodulators to further boost the activity of T cells, and examples of this include FAP-4-1BBL and CD19-4-1BBL fusion proteins as well as a PD1-targeted IL2v immunocytokine. With multiple engineered antibody projects in the clinical study phase, Roche is set to make big waves in the immunotherapy field.
2.) Alligator Biosciences: ‘Neo-X-Prime’
Alligator Biosciences is a Swedish-based clinical-stage biotechnology company entirely focused on immuno-oncology since 2008. Their pipeline includes novel mono- and bispecific antibody technology platforms and have been listed on the Nasdaq Stockholm exchange since 2016. Neo-X-Prime is a neoantigen cross-presentation-inducing bispecific CD40 antibody created by Alligator Biosciences to enhance tumour-specific T cell priming. It is a tetravalent format consisting of two CD40 binding domains and two tumour-antigens. The Fc part of the bispecific is a silenced IgG1 which, according to Ellmark, “is critical when trying to avoid any interaction with Fc gamma receptors”. The CD 40 agonist in this antibody depends on cross-linking to induce a strong immune activation, meaning that it will only activate CD 40 when it binds to tumour antigens simultaneously.
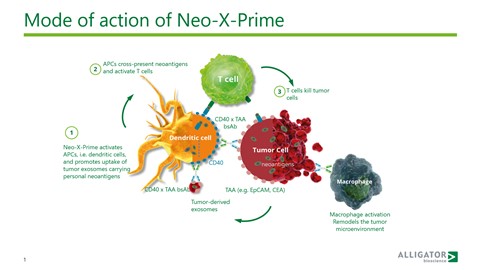
Alligator’s first-generation Neo-X compound is a technology platform designed and developed to enhance the priming, quantity, and quality of tumour specific T cells. Ellmark explains how “Neo-X provides an opportunity to drive the expansion of T cells which allows for treatment of indications that either has primary or secondary resistance to PD-1 and other checkpoint inhibitors”. Additionally, the CD40 antibodies act on myeloid cells and macrophages and can potentially remodel the tumour microenvironment. By mediating cancer activity, Neo-X activates dendritic cells and promotes the uptake of tumour exosomes carrying personal neoantigens. APCs then cross-present neoantigens and activate T cells which leads to the killing of tumour cells. CD40 was identified as the optimal DC target for inducing strong T cell internalisation and priming to tumour antigens with improved functional activity discernible when linked to the antigen.
3.) F-star Therapeutics: ‘FS222’
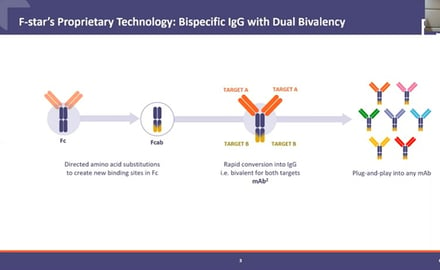
F-star is a clinical stage biopharmaceutical company founded in 2006 with an advanced pipeline of multiple immuno-oncology programs dedicated to creating a paradigm shift in cancer therapy. Its proprietary technology introduces mutations into the Fc domain of an antibody to then generate an Fc antigen-binding domain or ‘Fcab’. By subcloning the Fcab into a standard monoclonal antibody construct, a bispecific antibody, termed a mAb2 antibody, is established, which maintains the bivalent nature of the Fab arm and introduces the dual Fcab binding domains.
In the coming years, we can expect to witness new product launches globally and favourable policies implemented to aid in the fight against cancer.
FS222 is a clinical-stage programme and tetravalent bispecific antibody designed for optimal T cell activation with minimal toxicity. Ryan Fiehler, Associate Director of Discovery at F-star, stated that “the purpose of FS222 is to redirect activated T cells to the tumour”. FS222 simultaneously “releases the brake” on immune control of cancer by blocking the PD-1/PD-L1 pathway and “hits the gas” on immune activation by stimulating the CD137 pathway. The bispecific antibody also induces potent PD-L1-dependent T cell activation, and according to Fiehler, “shows strong anti-tumour activity in multiple models”.
What is most promising about FS222 is the potential to avoid dose-limiting hepatoxicity at therapeutic doses. In designing the technology platform, F-star established a mechanism of action that harnesses, as Fiehler explained, “the three C’s: crosslinking, clustering, and conditionality”. Resulting T- cell activation, proliferation, and eventual redirection occur to ensure tumour killing disease control. So far, F-star has found that FS222 bound CD137 and PD-L1 simultaneously and tetravalently to elicit potent in vitro T cell activation only in the presence of PD-L1. Studies using different valency combinations showed that tetravalent binding provided superior activity in terms of efficacy and potency in in-vitro assays. Therefore, FS222 presents a unique opportunity versus high affinity CD137 binding bispecific molecules by opening a broad therapeutic window.
Future Outlooks:
With a crowded pipeline, the bispecific antibody market will continue to grow at a double-digit rate. The rise in the number of cancer and chronic diseases, along with various government initiatives and cross-industry collaborations will see the field go (and grow) from strength to strength. In the coming years, we can expect to witness new product launches globally and favourable policies implemented to aid in the fight against cancer. At Oxford Global, we have our eyes firmly peeled and look forward to the latest developments as they unfold.