Bispecific T Cell Engagers and Synthetic Immunity: Circumventing Immune Escape
Presented by: Genevive Hernandez, Senior Director and Head of Clinical Biomarkers at IGM Biosciences
Edited by: Ben Norris
A serious obstacle in the treatment of cancers involves immune escape. Bispecific T cell engagers (TCEs) have been developed to mitigate this issue, forming a key aspect of the body's anti-cancer response. For Genevive Hernandez, Senior Director and Head of Clinical Biomarkers at IGM Biosciences, the aim is to convert non-specific endogenous T cells into synthetic anti-tumoural effectors. In achieving this, IGM Bio can work towards a longer-term goal of providing off-the-shelf therapies for cancer treatment and immunotherapy, with the end goal of synthetic immunity.
Circumventing Issues Around Synthetic Immunity
Genevive Hernandez’s presentation at Preclinical Immuno-Oncology 2022 began by touching on the different processes involved in the generation of endogenous, anti-cancerous T-cell-mediated immune responses. “There are various mechanisms which can limit each of these steps,” she opened. “These include the lack of an immunogenic tumour antigen, improper antigen presentation, no tumour-specific cells, or a low frequency of these cells.”
- The Main Challenges Facing Antibody-Drug Conjugates
- Enhancing T Cell Function with Small Molecule HPK1 Inhibitors: Express Your Cells
- The Applications of iNKT Cells in Immuno-Oncology
Additional complications can arise from the inhibition of T cell infiltration by tumour stroma, an immunosuppressive tumour microenvironment, and downregulation or loss of the major histocompatibility complex (MHC). Synthetic immunity is the means by which endogenous immune responses can be reprogrammed. “The idea behind synthetic immunity is to bypass these critical limitations and leverage the full repertoire of T cell syndication in order to kill cancer cells,” continued Hernandez.
There are two major approaches for inducing synthetic immunity: the first involves the transfer of T cells which have been genetically modified ex vivo to express Chimeric Antigen Receptors, better known as CAR-T cells. The second method concerns bispecific T cell engagers (TCEs). These methods are being piloted to assist in confronting immune escape — an immune scenario where new mutations of a particular pathogen may evade human antibodies.
Synthetic Immunity with Bispecific T Cell Engagers
As Hernandez explained, the goal with TCEs is to convert non-specific endogenous T cells into potent ‘synthetic’ anti-tumour effectors targeted at tumour-associated antigens (TAA). These are conditional, agonist recombinant antibodies, which are independent of both MHC and TCR. The aim of their application is to convert immune ‘cold’ to immune ‘hot’ tumours, with the end goal of providing off-the-shelf therapy with broad applicability and usage beyond transplant centres.
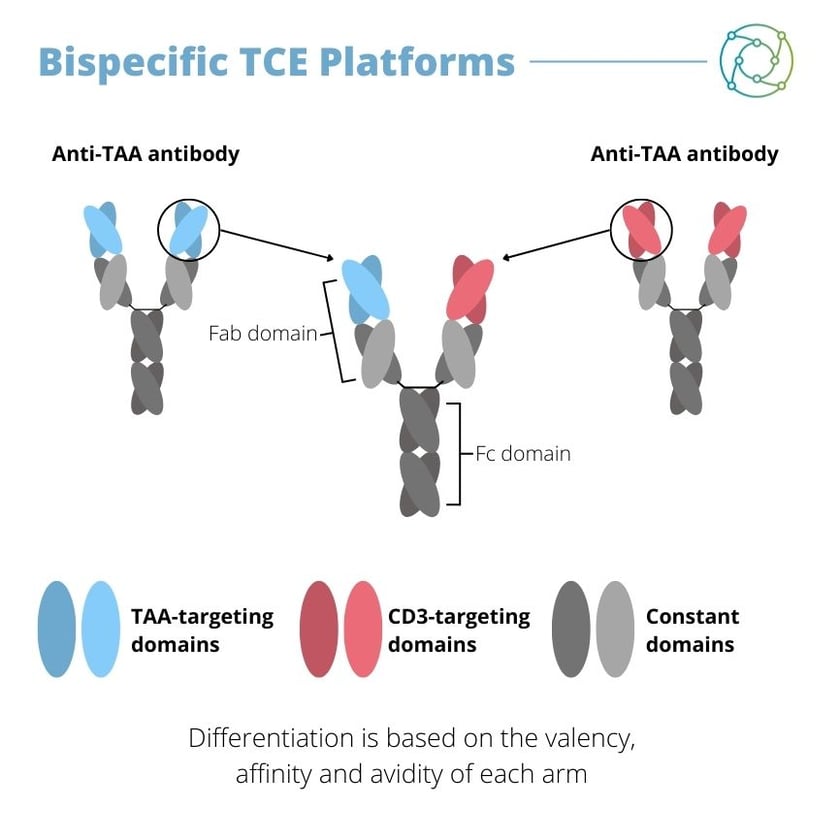
A major focus for Hernandez is bispecific TCE derived from recombinant antibodies. As the name denotes, these bispecific TCEs have two specificities: one is to a tumour-associated antigen expressed on the target’s cell surface, and the other is CD3 expressed on the T cell. “They act as a conditional agonist in an MHC, TCR and post-simulation independent fashion,” said Hernandez, “leading to a chance for the alteration of T cell specificity.” This subsequently triggers the conversion of non-specific endogenous T cells into potent, synthetic anti-tumour effectors that directly kill tumour cells.
At the time of Hernandez’s presentation, one TCE has been cleared for clinical use: blinatumomab, which was approved by the FDA in 2014 for the B cell precursor ALL. There are over 50 TCEs currently in clinical development worldwide, although right now the market is still maturing. The properties of antibodies concerned with TCEs can differ depending on characteristics such as valency and symmetry.
CD20xCD3 TCE Biomarkers Based on Mechanism of Action
Focusing on the specific example of CD20xCD3 TCE, Hernandez discussed some of the traits of these treatment methods in pre-clinical and clinical studies. TCE stimulation results in the activation of biomarkers CD69 and CD25, along with HLA-DR. This leads to T cell proliferation and secretion of cytokines, as well as the infiltration of T cells into tumour tissue. Hernandez emphasised that the quality and quantity of T cells is key, with a strong and rapid activation required from TCE for early tumour cell clearance. However, if the activation is too strong then this can lead to acute safety consequences, and this may result in a loss of T cell responsiveness.
With tumour cells, antigen expression is necessary but not always sufficient: proliferative status and susceptibility to apoptosis may counteract T cell cytotoxicity. At the same time, T cells need to be in the right location to function effectively. Biomarker data for bispecific TCEs shows that there may be many benefits for these drugs, with evidence that the number, location, and functionality of T cells may all be critical.
Next Generation Bispecific TCE: Engineered IgM Antibodies
Hernandez stated that one next generation approach to enable several levels of controlling TCE activity involves engineered IgM-based TCE that can bind irreversibly to cancer cells. “Because of the biophysical, stoichiometric and temporal features of this platform, we hypothesise that it can deliver a more physiological signal to T cells that also limits over-stimulation.” IGM-2323 (imvotamab) is a novel CD20xCD3 TCE with 10 high-affinity, high-avidity binding sites to CD20 and a single binding site to CD3 designed to enhance immune modulation.
IGM-2323 provides T cell-dependent and complement-dependent killing of cancer cells, and subsequently leads to repeatable IFN?? dominant immune action. “Importantly, IGM-2323 may limit the supraphysiologic stimulation of T cells,” she added. Its application may provide more physiologic T cell activation compared with existing T cell-engaging antibodies, and T cells respond differently to variable levels of TCR signalling. This leads to the secretion of more physiologic levels of cytokines, improving their safety and tolerability.
Synthetic Immunity Takeaways
Hernandez rounded off her presentation by emphasising some of the main takeaways for bispecific TCEs in terms of their applications. Synthetic immunity mediated by bispecific TCEs may help to bypass immune escape posed by a lack of appropriate immunotherapeutic target expression, MHC loss, and multiple layers of T cell regeneration by generating the potent T cell-based killing of cancer cells.
Some of the biggest challenges for the further development and implementation of bispecific TCEs include cytokine release syndrome (CRS), target expression levels, T cell function in the tumour microenvironment, and its impact on endogenous immunity in the host. As Hernandez explained, bispecific TCE activity can be monitored through select host biomarkers. Based on the mechanism of action (MOA), biomarker behaviour can inform the selection of dose and schedule, the mitigation of safety risks, and the enhancement of efficacy.
Hernandez thanked her colleagues at IGM Biosciences for their work on IGM-2323 (imvotamab), as well as the patients, caregivers, investigators, and nurses for their studies.
Want to read more about new advances in immuno-oncology and ongoing immune therapies? Visit our Immuno portal to find current insights from the industry’s best and brightest. If you’d like to register your interest in our upcoming Tumour Microenvironment: In-Person conference, visit our event website.
Speaker Biographies
Genevive Hernandez-Pantua is Senior Director and Head of Clinical Biomarkers at IGM Biosciences, a biotechnology company pioneering the use of novel engineered multivalent and multispecific therapeutics. She was previously at Genentech, where she completed her postdoctoral studies on the interplay between angiogenesis and tumour immunity, and then became the Biomarker Lead for multiple trials of the breakthrough cancer immunotherapies atezolizumab (anti-PDL1 Ab) and mosunetuzumab (CD20xCD3 bispecific Ab).
Related Resources