Exploring & Developing Various Biotherapeutic Modalities

In October 2022, Oxford Global’s Biologics series was delighted to host a panel of industry experts for the Discussion Group, Exploring & Developing Various Biotherapeutic Modalities. The group came together for one hour of conversation and debate on overcoming challenges in analytical method development and accelerating the future of biotherapeutics.
Meet the Panel

Galahad Deperalta, Senior Scientist and Innovation Group Leader at Genentech, moderated the session. With over 21 years of early and late-stage biotherapeutic drug development experience, Deperalta has an in-depth knowledge of process development, GMP/QC analytical methods, protein chemistry, and regulatory filings. As Innovation Leader, his main priorities included data and digital transformation solutions and digital modelling.

Joining Deperalta was Paul Guyett, Group Lead of Innovation at Invenra. Guyett has 11 years experience in drug discovery and currently leads a team focussed on maximising the value of their bispecific antibody platform. Whilst Guyett has been dedicated to discovery efforts, he is “excited” to explore new biologic modalities based on desired biologic efficacy.

Slobodanka Dina Manceva, Associate Director at Teva Pharma,has spent 17 years working in early and late-stage drug product development on a broad range of biologic modalities. Developing high throughput formulation platforms, streamlining data analysis and collection, and developing artificial intelligence (AI) and machine learning (ML) algorithms are integral to her role at Teva Pharma. Manceva describes her professional interest as being “addicted to finding ways of overcoming the challenges of developing and working with new modalities.”

The final panel member to co-lead the discussion was Smita Raghava, Associate Principal Scientist at Merck. As co-author of eight patent applications and 20 research articles, including book chapters and postdoctoral research work (at UMass Amherst), Raghava has more than 10 years experience working in biotherapeutics drug development. In particular, she specialises in biologics high-throughput pre-formulation screening, and early & late stage formulation development. Raghava also has experience working with drug product manufacturing, process optimisation, and the characterisation of biotherapeutics.
Exploring Different Biological Modalities
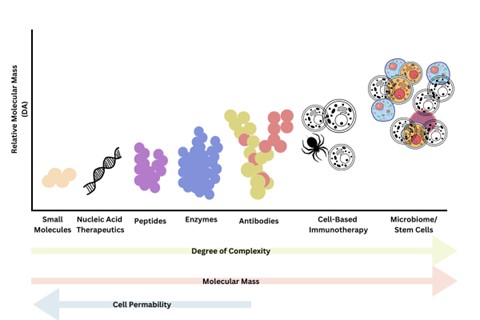
The session began with a brief overview of the different biological modalities available. Biologic modalities range from small molecules all the way up to microbiome-based biologics and stem cells (see figure. 1). Biological products can comprise of different cellular components, such as chemical messengers like cytokines, interferons, nucleic acids, proteins, and even whole cells or cells present in the microbiome tissue organisation. Raghava pointed out that “as the size of biologics increase, there is an increase in the complexity and difficulty in handling”.
Currently, biotherapeutic modalities have proven to be effective drugs and have shown success in treating a wide variety of diseases and disorders. Unlike conventional chemically synthesised drugs, they exhibit higher specificity and low toxicity and thereby show more efficacy than small molecule drugs.
The full potential of biologics is causing a lot of excitement in the industry right now. “Pharma and biotech companies are busy focusing on the research and development of next-generation biologics with the aim of achieving enhanced characteristics and improved pharmaceutical properties,” Raghava said. “We can expect to see biotherapeutic modalities cropping up in almost every company’s pipeline,” she predicted.
Accelerating the Development of Next Generation Antibody Therapeutics
In response to Raghava’s comments, Manceva went on to claim that “biotherapeutics are an important part of the current movement in the health sciences field and particularly in drug development.” The number of biotherapeutics approved in the EU and US rapidly increases yearly.
“As the size of biologics increase, there is an increase in the complexity and difficulty in handling."
The first monoclonal antibody approved by the FDA was Muromonab in 1986. As of September 2nd, 2022, 19 investigational antibody therapeutics are in regulatory review in the US and EU. “Interestingly, many of these emerging drug candidates are not restricted to cancer-related molecules; there is a lot of development happening in the non-cancer field as well” Manceva explained.
Guyett continued by stating that “not only is there tremendous variety in the types of biologics, everything from cell-based therapeutics to nucleotide therapies, but even within the antibody space, we're seeing a growing diversity of utilisation of the immunoglobulin fold.” When it comes to accelerating the development of next-generation antibody therapeutics, Guyett sees the need for a “plug and play” approach.
- Analytical Method Development for Biotherapeutics
- Widening the Therapeutic Window: Development of Antibody Drug Conjugates for Solid Tumours
- Bispecific CAR-T Cell Therapies: Successful Applications in Mouse Tumour Models
There has been so much study of the fold itself; its structure function, how you develop it, and how you manufacture it, is where we are reaching a plug and play point,” he explained. In other words, “biologists are successfully hitting that much-desired efficacy and creating easy-to-use, highly functional therapeutic antibodies.” Guyett observed that the progress being made in the development of next-generation antibody therapeutics has meant that many companies are now looking to develop epitopes de novo via computer machine learning (ML) and AI (artificial intelligence) strategies.
Addressing Developability Challenges
One of the critical questions arising from the discussion was the potential difficulties of accelerating developability. Deperalta asked, “what are some of the challenges associated with achieving that next generation of biotherapeutics?” Some audience members drew attention to a heavy reliance on engineered immunoglobulin folds. This is because there may be development risks associated with linker chemistries and conjugations.
Stability is a further issue to be aware of when developing next-generation biotherapeutic modalities. In particular, nanobodies and bispecific antibodies may suffer from compromised stability. Molecular modelling offers one solution to help address the issue, as well as AI and ML technology. To facilitate computerised solutions, inevitable investments in bioinformatics data algorithms and storage will be necessary.
What’s Next in the Discussion Group Series?
We will continue our Discussion Group series in November with a session focusing on Universal Vaccines & Vaccine Therapeutics, which will be led by Mark Doherty, Senior Medical Manager at GSK. To gain exclusive access to the upcoming sessions in our Biologics series, click here to find out more about our membership offerings.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.