The Bispecific Antibody Landscape
Multispecific antibodies are engineered proteins that can bind to multiple targets simultaneously. They can be designed to recognise and bind to two or more different antigens, receptors, or cells with high specificity and affinity. Bispecific antibodies are a subset of multispecific antibodies as they can only bind to two targets simultaneously. However, they still have enormous potential as new therapeutic strategies by enhancing the specificity and potency of antibodies.
Research into bispecific antibodies is still expanding, and their delivery and capabilities have continued to be explored. In this insight article, we look at some presentations from Oxford Global’s Biologics 2023 event, which discuss these new delivery avenues and how we can increase our understanding of bispecific antibodies’ pharmacokinetic profiles.
Presentation 1: BEPO® for the Long-Acting Delivery of Proteins
Presented by: Andrea Gonella, Senior Research and Innovation Associate MedinCell
MedinCell has developed BEPO® drug delivery technology, an injectable technology which can deliver both small molecules, peptides, and proteins. The core components of this technology are the active pharmaceutical ingredients, a solvent, and copolymers. Co-excipients may also be added depending on the type of molecule being delivered. Upon its injection, the polymers of the BEPO® technology precipitate due to a phase separation mechanism and encapsulation of the molecules in depots occurs. The molecule is then released in a controlled manner for the required time.
In term of manufacturing, it overcomes many of the drawbacks observed with classical long-acting injectable technologies used to deliver fragile molecules, such as mechanical stresses or high temperatures. The proteins are mixed with some excipients to improve stability and release kinetics. The protein is then spray-dried and mixed with the vehicle (composed of copolymers and solvent). Upon mixing, an injectable suspension is obtained. The BEPO® technology can be used to deliver drugs both systematically and locally.
Gonella described a case study to demonstrate the potential of BEPO® to deliver a bispecific antibody. The bispecific molecule, BiJ591, was designed along the BiTE® scaffold, using the scFvs of two antibodies, one which targets the prostate membrane antigen PSMA, and one the CD3 receptor of T-cells.
A binding assay determined that the formulation approach did not impact the protein's binding capacity, and in vivo pharmacokinetic profiles showed that the delivery of the molecule could be sustained for up to 21 days.
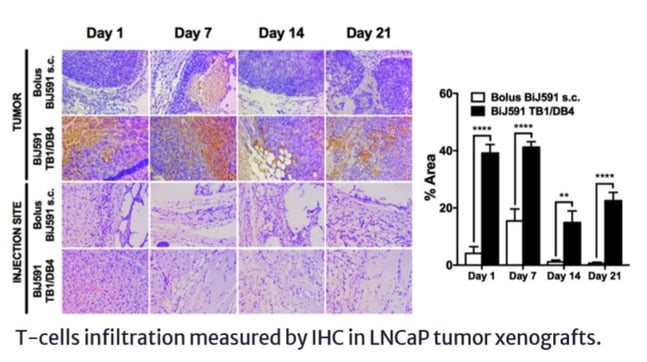
The BEPO®-loaded bispecific’s anti-cancer activity was assessed with tumour-grafted mice. The mice were injected with human’s PBMCs (containing T cells) and, after two hours, the BEPO®-loaded formulation was injected (the vehicle and the free bispecific antibody were used as negative and positive controls, respectively). The tumour growth of the group injected with the bispecific administered using the BEPO® technology was better controlled compared to the one of the other groups.
The T-cells infiltration at the tumour and injection sites was also evaluated. When the bispecific was injected using BEPO® technology, much higher quantities of T cells were found at the tumour site than at the injection site (see Figure 1). Gonella commented that this reflected that the T cells were working “as they should, where they should.”
The capacity for BEPO® technology to control the regular delivery of a drug at the required therapeutic dose for the desired period is highly impressive. Moreover, its proven efficacy with bispecific delivery also enhances the therapeutic capacity of bispecific antibodies, marking an exciting step forward in the field.
Presentation 2: Emerging Mechanisms Influencing the Disposition & Clearance of Bispecific Antibodies
Presented by Amita Datta-Mannan, Associate Vice President at Eli Lilly
Whilst diversification via protein engineering has effectively expanded the structural landscape for bispecifics, mechanisms influencing the pharmacokinetic profiles of these structures are still broadly not understood. Moreover, fusion-based bispecific antibodies can display poor and unpredictable pharmacokinetics. This can cause aberrant clearance and pharmacokinetics, leading to poor drug ability, increased toxicity, and potentially more significant immunogenicity risks.
Datta-Mannan's presentation evaluated the influence of the neonatal Fc receptor (FcRn) interactions on the pharmacokinetic properties of two bispecific antibody formats with different fusion partners. These case studies aimed to understand the non-target-based factors that influence the disposition and clearance of bispecific antibodies.
- AI-Discovered Peptide has Dramatic Impact on Muscle Conditioning
- World Malaria Day 2023: Breakthroughs in Malaria Vaccinations
- Tiny but Mighty Molecular Syringe Developed Using AI
In the first case study, scFv fusion constructs were appended to human IgG4 C-terminal HC. Two bispecific constructs were created with the same target binding, but the orientation was switched with different monoclonal antibodies (mAb). Bispecific A's clearance was the same as the parental antibody, while bispecific B’s was seven times faster than the parental mAb. The bispecific fusion negatively impacted the pharmacokinetics and was deemed unacceptable for further development.
Researchers assessed the in vivo distribution of the fusion-based bispecific antibodies by associating them with clearance organs in cynomolgus monkeys. Datta-Mannan explained that the rapid clearance of bispecific B was related to increased intracellular degradation within tissues, as the bispecific itself showed no enhanced association with any one tissue.
There was no difference in physical, chemical, and thermal stability between the two bispecifics, leading researchers to investigate further the cause of the differential pharmacokinetic findings. They established that the antibodies displayed a difference in FcRn release and concluded that aberrant FcRn release at neutral pHs was responsible for the varied pharmacokinetic profiles.
The second case study investigated the pharmacokinetics of protein fusion-based bispecific antibody constructs. A protein domain was fused to the IgG4 heavy and light chains via a Gly-Ser linker. Of the bispecifics investigated, only that which had the protein fused to the N-terminal end of the heavy chain had pharmacokinetic profiles similar to the parent mAb.
However, constructs where the protein fused to the C-terminal heavy chain or N-terminal end of the light chain had a substantially higher clearance than the parent antibody. Further investigation into the cause of the rapid clearance revealed that, unlike the first case study, this property was due to increased tissue association, with these bispecifics exhibiting increased liver binding.
Researchers also established that the bispecifics’ configuration only marginally influences their in vitro physiochemical developability factors. The only notable aspect found when the physical, chemical, and thermal stability of molecules was tested was that the bispecifics which performed poorly had a marginally longer retention time, suggesting increased hydrophobicity-based interactions, which may have led to negative pharmacokinetics.
Overall, fusion-based bispecific antibodies can display complex pharmacokinetics and biodistribution, which is influenced by both the fusion partner's structure and the fusion position's configuration. Datta-Mannan concluded by noting that rational bispecific antibody design is a crucial parameter for optimal in vivo clearance and disposition. An increased focus on this aspect will lead to the design of more effective bispecific antibodies in the future.
Join Oxford Global’s annual Antibody Engineering 2023: Online event today. This intensive 2-day meeting delves into the latest in antibody engineering and antibody-based therapeutics & a meeting place for experts working within engineering, computational tools and antibody-based therapeutics. Download the agenda to join prominent leaders and scientists as they share new case studies, innovative data, and exciting industry outlooks.