Widening the Therapeutic Window: Development of Antibody Drug Conjugates for Solid Tumours

Presented by: Mahendra Deonarain, Chief Executive and Science Officer, Antikor Biopharma Ltd
Edited by: Ben Norris
ADCs – antibody drug conjugates – are complex therapies for cancer treatment which are intended to target and kill tumour cells while sparing healthy cells. As Mahendra Deonarain, Chief Executive and Science Officer at Antikor Biopharma Ltd told the audience at Oxford Global’s Biologics UK: In Person 2022 conference, ADCs have really come of age in the past five years. Much of the current focus in treatment is on operability against solid tumours in breast, cervical, or urothelial cancers. “Expansion to other solid tumours is what will drive more investment into our area,” said Deonarain.
Antibody Drug Conjugates: Modified Payloads
“There are so many great antibody engineering or chemical modification designs to improve ADCs,” Deonarain began. These modifications range from minute details such as payload release time, payload type, and conjugate affinity. Most ADCs which are ultimately approved for use are effective against non-solid tumours. Solid tumours are more difficult to address, and currently the main cancer types which ADCs have been approved for are breast or cervical/urothelial cancers. As such, other indications – such as lung, gastric, and colon cancer – are still not being successfully addressed with these treatments. Deonarain has subsequently identified ADCs which tackle these types of difficult solid tumours as a key area of focus.
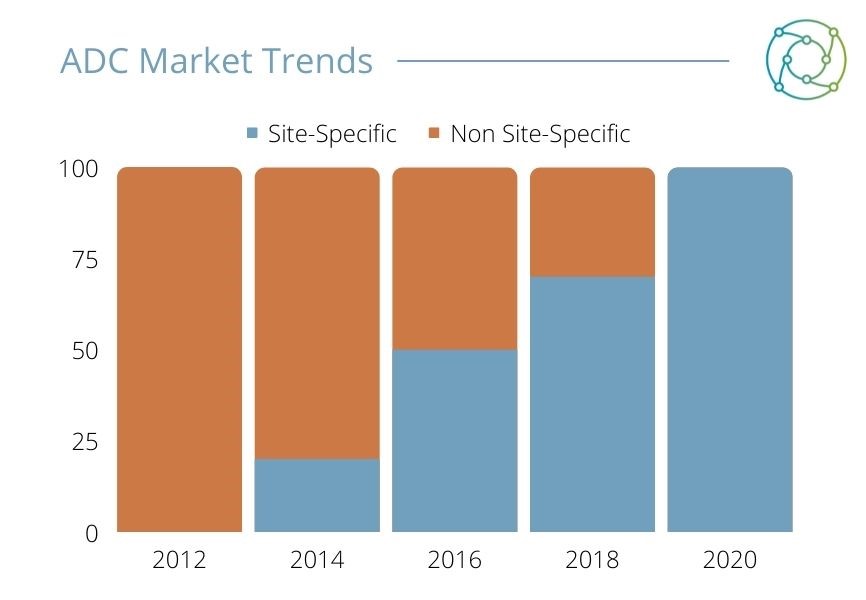
Investment in ADCs in recent years has passed the threshold of transforming the treatment into a commodified industry (see Figure 1), increasing massively since ADCs first reached the market in 2011. A drive towards site-specific conjugations – where antibodies are coupled with cytotoxins to provide homogenous conjugates – has driven the development of better-quality products in the ADC community, although the present market is still dominated by linkers based on peptides. “There are many new technologies now coming online where you conjugate sites specifically,” said Deonarain.
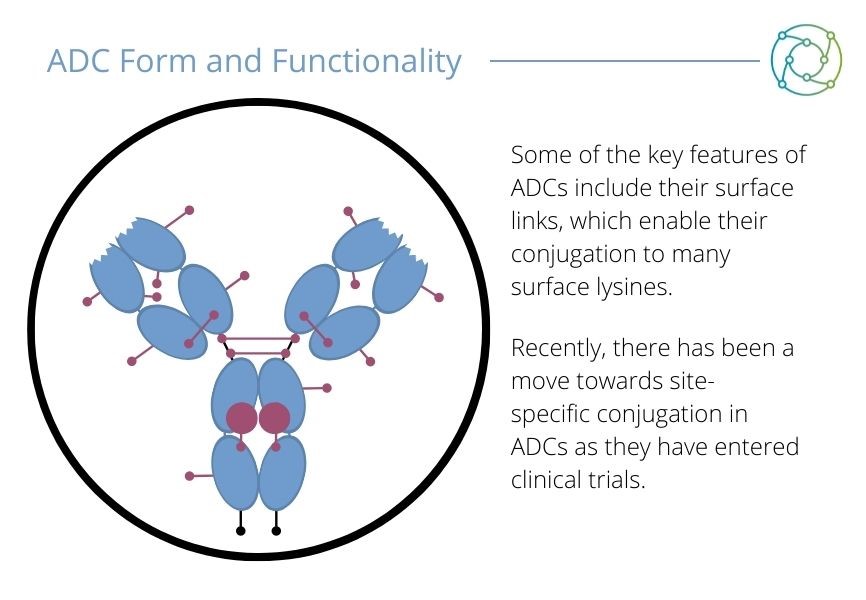
Deonarain emphasised the importance of considering the ADC as a whole, including the linker and payload (seen in Figure 2). “In terms of linkers, there are two areas: cleavable linkers and non-cleavable linkers.” He continued that cleavable linkers were the variant currently dominating the market, as non-cleavable linkers had not been thoroughly taken up by the industry. Payload delivery has been a “source of angst” in the ADC community for some time, as Deonarain explained. Clinical trials are dominated by micro-tumoural inhibitors, while more recent entrants are developing other payload classes with a different method of action.
Treatment Tolerability for Solid Tumours
As Deonarain highlighted at the start of his presentation, the current focus in ADC research is on obtaining better results in solid tumours. “With ADCs, we always talk about the therapeutic window or therapeutic index as being what we need to address. Not just the potency, not just the safety, but the two things together.” The therapeutic index (TI) is a quantitative assessment of a drug’s safety proportionate to its effectiveness. There is a threshold in treatments for cancer beyond which the damage done to the patient’s body and immune system outweighs the benefit of the tumour-killing properties of that treatment.
“What we want to do is widen the therapeutic window of tolerability versus efficacy."
Since solid tumours require higher concentrations of ADCs than non-solid tumours, finding an effective treatment which falls within the threshold of tolerability is more difficult. Deonarain discussed the TI in the context of an idealised ratio between the maximum tolerated dose and what should be the minimum effective dose. “What you want to do is widen that window.” Less potent but better-tolerated doses can drive treatment penetration into solid tumours, whereas high-concentrations of potent ADCs are more challenging for patients to tolerate. As an additional complicating factor, the scarcity of solid tumour ADCs makes it difficult to obtain sample data to incorporate into future studies.
HER2 Functionality and Delivery
Discussing less harmful treatment methods, Deonarain moved on to ADCs which target HER2 – a protein called human epidermal growth factor 2 which promotes the growth of cancer cells. He identified ADCs with a cleavable linker and an deruxtecan payload as a potential solution. “In terms of potency, it’s well-tolerated, because the payload classes have a sort of nanomolar potency,” Deonarain said. He discussed the clinical results of trial treatments for patients with breast cancer, asserting that Enhertu outperformed other standard of treatments for breast cancers.
As well as possessing a different method of action, it can be administered at a higher dose and possesses a different method of action in terms of the payload. “It’s the same drug,” said Deonarain, “but with different tolerability, different indications and a different level of penetration.” However, during trials in patients with gastric cancer the results are very different, with progression-free survival for patients only registering at five months, compared to over 28 months for breast cancer. “There are many complicated things to try and tease out,” said Deonarain, “including the reason why it’s superior in breast cancer compared to gastric cancer.”
- Novartis Withdraws Non-Small Cell Lung Cancer Monotherapy
- Measuring Bactericidal Antibody Response: Fighting Global Morbidity
- Avoiding Roadblocks to Treatments for Tumourous Tissues
Fundamentally, one of the most important factors in the successful application of an ADC is penetration, which Deonarain identified as a complicated issue. “Penetration requires a high concentration of product in the plasma. Additionally, it has to overcome many barriers trying to get into the tumour.” He added that interstitial pressure was a key barrier to penetration. “That will bring into play the density of the receptor and the ADC half-life, as well as how quickly it’s internalised – because if it’s internalising too quickly it’s not going to penetrate.”
Future Developments for ADC Penetration
Strangely, using a cold/unconjugated antibody to drive ADC penetration could be one workable approach to improve treatment and safety without compromising patient health. Deonarain said that for ADCs, this could be the missing piece of the puzzle to get better efficacy in solid tumours. “There are so many antibody formats available,” said Deonarain to wrap up his presentation. Smaller formats like antibody fragments could be the answer, but some antibody fragments may not work conclusively.
Antikor’s approach shows that there is still scope for research and innovation in this space. Having arrived at the fourth generation of ADCs for solid tumours, there is a now a contrasting approach of slow uptake with slow clearance compared to rapid uptake with rapid clearance. “To successfully apply antibody fragments as drug conjugates (FDCs), we have built our own single-chain Fv antibody libraries,” added Deonarain as he rounded off, “and we really focus on high payload ratios and product developability because you really don’t want conjugates to aggregate as this compromises both efficacy and safety.”
Visit our Biologics portal to find out more about the latest developments in therapeutics and contemporary healthcare. To register your interest in our upcoming Oligonucleotide Chemistry & Therapeutics Symposium, click here.