Using Humanised Mice to Evaluate Vaccine Efficacy
When we talk about vaccines, we refer to a wide variety of different modalities. Nevertheless, they all abide by the same principle: to stimulate the production of antibodies to provide protective immunity against infectious diseases. Historically, the most popular and well-known vaccine is the seasonal flu jab. This takes the form of an inactivated injectable, which contains whole bacteria or viruses that have been killed or altered, so they cannot replicate.
Other well-known modalities include the live attenuated format, which involves injecting a weakened living virus to generate a protective immune response. This is achieved through genetic modification of the disease pathogen, with examples including the polio and the chicken pox vaccine.
- Why We Develop Autoimmunity: Hyperstimulation of Genetically Prone Subjects
- Advanced Vaccine Platforms & Technologies
- Pfizer’s Pneumococcal Vaccine Shows Great Promise in European Study
The Subunit format, currently used in the hepatitis B virus vaccine administered during infancy, relies on purified and intact parts of the infected organism being injected to stimulate immunity. Alternatively, the meningitis vaccine recently developed by GSK contains four different components of the surface bacteria Neisseria meningitidis.
The vaccine that has reached unprecedented recognition to date is the SARS-CoV-2 — or — mRNA modality. Proposed by Pfizer-BioNTech, the messenger RNA (mRNA) vaccine format uses genetically engineered mRNA to programme to produce the S protein found on the surface of the COVID-19 virus. In response, the body creates antibodies which become activated should someone become infected with the virus.
“The platform allows us to study the human immune response in great detail, and as such provides the opportunity to test the efficacy of complex vaccines,” Kymab confirmed.
Despite the diversity of vaccine modalities available to both researchers and clinicians, all formats have one thing in common; they induce an antibody response against infections. Antibodies are the only proven correlates of vaccines and are essential to the research and development of technology developments. During their presentation at Oxford Global’s Biologics UK 2021 conference, Kymab asserted the necessity of complex antigen study to refine the repertoire of available vaccine platforms using humanised mice models.
Kymab: Maximising Vaccine Development Efforts
Kymab Ltd is a Cambridge-based Sanofi company that focuses on developing novel human antibody-derived therapies for a broad range of indications (see figure. 1). In proposing unique transgenic platforms, Kymab’s vast portfolio of fully human, species cross-reactive, and affinity-matured antibodies work to fight against challenging targets. “We are one of a number of companies to develop transgenic platforms in mice to generate human antibodies,” Kymab explained.

“At Kymab, we also have the transgenic capability to generate to an immunogen, given to a mouse, that are chimeric in nature,” Kymab continued. This means they contain human variable heavy and light chain sequences that bind to the FC domain of the mice. With investment and backing from the Bill & Melinda Gates Foundation, Kymab has launched active programmes to test the efficacy of research-stage vaccines and identify therapeutics antibodies targeting pathogens.
Human Immunoglobulin Repertoire Mice in Vaccine Development
The utility of Kymab’s transgenic platforms is centred on its ability to provides a comprehensive pipeline and process for studying complex antigens (see figure .2). “The platforms have enabled us to put both licensed and early-stage development vaccines into mice to study a human response to infectious disease,” they confirmed.
A profound benefit to using mice models is that regulatory approval is not needed when administering vaccine candidates to mice. Kymab described this advantage as having a “profound impact” on clinicians' ability to study the human immune system. Human immunoglobulin mice models are a translatory tool that measure human immune activity and facilitate greater experimentation and investigation during vaccine development.
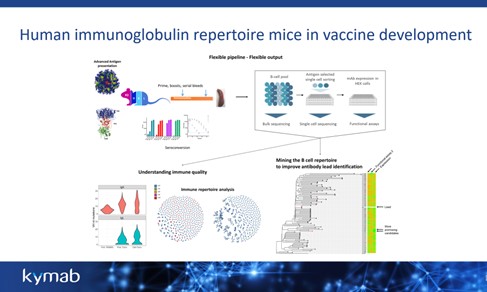
Figure. 2 Kymab’s transgenics platforms are designed to generate fully-human monoclonal antibodies from highly-engineered strains of mice that have the complete constellation of human antibody building blocks in their genome.
“What is wonderful about mice models is the fact that you don’t need a human to study human antibody responses,” they said. Antibody testing in humans is strictly related to blood testing, whilst humanised mice provide the opportunity to test primary and secondary lymphoid tissues such as bone marrow and the spleen. In mice models, the capabilities for vaccine regiments, such as prime booster administration, is close-to unlimited.
Understanding Immune Quality of Complex Vaccines
“The platform allows us to study the human immune response in great detail, and as such provides the opportunity to test the efficacy of complex vaccines,” Kymab confirmed. By running bulk sequencing of B-cells, Kymab’s platform delivers information about the breadth of heavy and light chain sequence variability. It is then possible to express these chain sequence pairs in various formats to study the function of individual antibodies induced.
“Having a light chain paired sequence is integral to understanding the immune quality and in turn, vaccine efficacy,” Kymab pointed out. Using affinity maturation, clinicians can determine the relationship between the number of mutations in the differing chain regions and complementarity-determining regions (CDRs). This informatics method interprets and measures the breadth of the effective response from a complex vaccine.
The Proof is in the Platform: Preventing Acinetobacter Drug Resistance
Kymab’s platform has successfully demonstrated the utility of understanding immune responses to bacterial vaccines in humans. In particular, Kymab’s interest in antimicrobial resistance has helped to unlock invaluable insights into one of the most complicated bacteria: Acinetobacter baumannii. “We are standing at the end of the modern antibiotic era,” they confirmed — “there is a large number of bacteria that is becoming resistant to antibiotics, and this is becoming problematic, particularly in hospital settings.”
Acinetobacter baumannii is notorious for picking up resistance against antibiotics, and globally speaking, has been recognised as the most difficult bacterium to treat. With the help of Kymab’s transgenic mice platform, researchers were able to work towards the development of monoclonal antibody-based therapies to provide “instant immunity” within the hospital setting. During the presentation they described the outcome as “a cocktail of monoclonal antibodies that replicate protective immunity normally afforded by immunisation.”
Looking ahead, Kymab will continue working with their investors to exploit further their cutting-edge platform's discovery and development capabilities to fuel future pipelines. Whilst its core areas of interest are autoimmunity, oncology, and immuno-oncology, Kymab remains open to exploring targets across other disease areas.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.








