Using Cancer Organoids to Discover New Therapeutic Targets
Presented by: Étienne de Braekeleer, Senior Research Scientist at AstraZeneca
Edited by: Cara Digby-Patel
The number of predictive models available from cancer cell lines is currently very limited. This lack of models massively restricts researchers' capacity to discover new therapeutic targets for cancer treatment. But how can these limitations be overcome?
Étienne de Braekeleer, Senior Research Scientist at AstraZeneca, has been working to establish the benefits of using organoids to produce new lung models to help better predict the clinical success of candidate drug molecules. At Oxford Global's 3D Cell Culture Online symposium in 2022, de Braekeleer discussed what organoids are, their benefits, and how they can be used to understand the tumour microenvironment better.
Organoids 101: What They Are and Why They're Used
Organoids are complex and well-organised 3D structures which keep the heterogeneity of the patient tissue used to create them. Grown in 3D organisation, apoptotic cells are shed outside the growing organoid, allowing the structure to maintain its flexibility and the complexity of the original tissue.
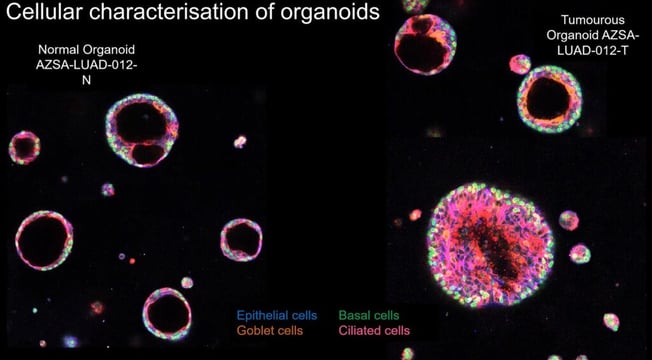
At least, four distinct subcellular subtypes are found within organoids: epithelial cells, basal cells, goblet cells, and ciliated cells. What is particularly striking when looking at the cellular characterisation of organoids is the variation in structure between normal organoids and tumorous organoids. The former is very well structured, with a distinct layer of epithelial cells and basal cells, with goblet and ciliated cells found inside the organoid forming the lumen. By contrast, this well-defined structure is entirely lost in the cancer organoids (see Figure 1).
The main benefit of using organoids is the success rate of initiation; when tissue is taken from patients and derived into organoids, the success rate is much higher than it would be if they were derived from cell lines or if attempts were made to transplant them into patient-derived xenografts.
Organoids are also substantially easier to biobank compared to these alternative methods. The fact that the heterogeneity of patient tissue is maintained means that cancer organoids retain most of the phenotypic and genetic features of the original patient tissue.
De Braekeleer described how AstraZeneca, in collaboration with the Royal Papworth Hospital, the Wellcome Sanger Institute, and the Human Cancer Models Initiative, built new lung models to overcome the pre-existing lack of predictive models for a variety of mutations.
Understanding the Tumour Microenvironment
To better understand which features of a tumour can be retained in cancer organoids, researchers performed various assays to gain a hierarchical understanding of the tumour microenvironment (TME).
Using cytometry time of flight (CyToF) or imaging mass cytometry (IMC) , researchers established tissue architecture, immune deconvolution, and the phenotyping of the TME. All these different elements provided a better understanding of how the cells interact with each other and with the TME.
This used tissue slices stained by metal-labelled antibodies, analysing up to 32 metal-conjugated antibodies simultaneously, with a spatial resolution as small as 1?m.
- Apical-Out Organoids: A Novel Strategy to Control the Polarity of Organoids Closing
- Understanding the Capabilities of 3D Bioprinting for Cell and Gene Therapy
- Human Stem Cell Model Development for Neurodegenerative Diseases
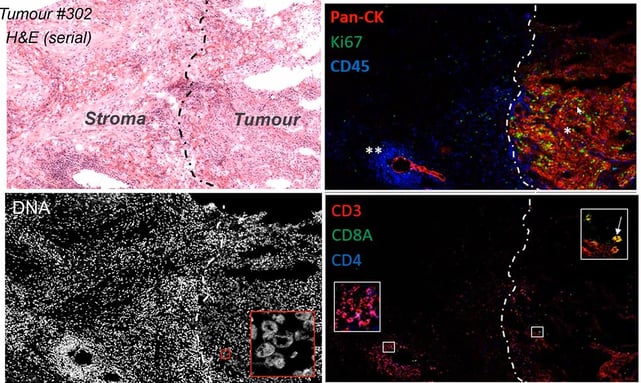
IMC meant that it was substantially easier to assess the deep cellular phenotyping of human tumour tissue and to discern tumour tissue from stroma, compared to standard staining. By looking at different parameters such as PanCK and Ki67 levels, CyToF reflected the tissue proliferation (see Figure 2). It also revealed that most immune cells were located at the invasive front of the tumour, whilst other cell types were either located around the vascularisation or had infiltrated the tumour.
Mass spectrometry imaging (MSI) is another technique used to gain more information about the tumour microenvironment. By scanning tissue slices with an ionising source, researchers determined the ion density at different positions. These readings can then be merged into ion density maps to see the interaction of a range of metabolites and observe the variations between different parts of the tissue.
De Braekeleer noted that if several types of tissue were measured and clustered, a specific metabolic profile for cancer tissues was identifiable, which was surprising as this happened across patients despite variations in each individual case. The primary advantage of these two techniques is that IMC can be integrated into the MSI to create an “immuno-metabolism profile,” which illustrates how a variety of metabolites interact with the TME.
Researchers were able to perform MSI on cancer organoids, and they established a specific metabolic profile for tumour organoids compared to non-tumour organoids. Unsurprisingly, metabolites associated with the TME, such as lactate, appeared in far greater quantities in tumorous organoids.
Organoids for Immuno-Oncology Studies
So how much of the immuno-metabolism profile can actually be translated from tumour tissues into organoids? Unfortunately, the answer is currently not much at all, because the growth conditions for tumour tissue compared to organoids vary substantially. The latter are grown in normoxic rather than hypoxic conditions, meaning that the media used has much higher oxygen levels. This therefore significantly alters the metabolites which are produced and secreted.
Nevertheless, the data from the assays described can still be used to produce better living therapies. In one subsequent study, organoids were embedded with primitive primary T cells, and CAR-T cells were added. The CAR-T cells had no difficulty infiltrating the organoids and slowly killing them.
However, if primitive T cells were replaced with T regulatory cells, CAR-T infiltration slowed significantly, and those CAR-T cells which did enter the organoid did not do much damage but simply circled the structure.
This assay reflected that many parameters within the TME limit the efficacy of different therapies. De Braekeleer closed by noting that the next big step will be utilising CRISPR screens in cancer organoids to identify and validate new therapeutic targets. Ultimately, he said, “organoids are revolutionising our understanding of cancer heterogeneity and its implications for better therapeutics,” and expressed hope that in the future, organoids will be more widely adopted so that this therapeutic discovery process will be rendered more efficient and effective.
Accelerating the adoption of 3D models in pre-clinical and translational research features as a key topic area of our next-in-series event, 3D Cell Culture 2023. Happening from 25th to 26 th April 2023 in London, UK, the programme agenda will be bringing together industry influencers to drive 3D model implementation and development for predictivity, validation, safety, and toxicity studies. This event is co-located with Organ Modelling UK: In-Person.