Tackling Solid Tumours in Immuno-Oncology
Day one of Oxford Global’s Online Immuno Week 2021 brought us an especially engaging panel discussion about the advances of immuno-oncology for treating solid tumours. This insight article will cover the highlights and challenges that our panellists have encountered during their work. We hear about an innovative new technology for increasing the viability of CAR T therapy, as well as perspectives on the development of combination therapies.
Treating HER2+ CNS Metastases with CAR T Engagers
In recent years CAR T cell therapy has taken the spotlight as one of the most promising treatments in oncology. May 2022 marked a decade since Emily Whitehead, the first paediatric CAR T patient, was declared cancer free. Whitehead was just six years old when she was diagnosed with acute lymphoblastic leukaemia. Now ten years later, she is happy and healthy. More than 10000 patients worldwide have received CAR T-cell therapy in a clinical trial, and there are now more than 5 approved products for hematologic cancers.
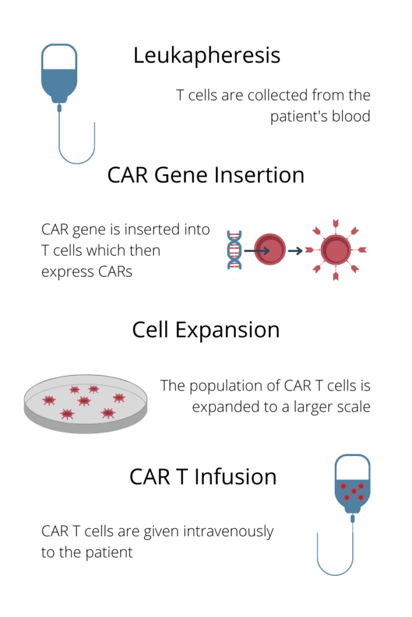
Infographic: The basic process of CAR T therapy
Due to the therapy’s immense capacity in blood cancers, researchers are now setting their sights on translating CAR Ts to solid tumours. Paul Rennert is Co-Founder, President, and CSO of Aleta BioTherapeutics, a cell therapy company that provides a platform technology that they call ‘CAR-T Engagers’. Their platform is intended to enhance CAR T therapy by using these engagers. Rennert claimed that Aleta’s efforts so far have acted as a “proof of concept for the treatment’s safety and efficacy.”
Rennert described how Aleta’s CAR T Engagers target the HER2 protein, a receptor which is present on some types of breast cancer. Rennert and his team are hoping to use their CAR T engagers to treat CNS metastases that express the HER2 protein. This is because the traditional biologic therapy for HER2 positive disease is not able to cross the blood–brain barrier.
This is where CAR T therapy would be valuable. Rennert explains that “the clinical data from CARs to CD19 tells us that those CARs traffic throughout the body, including into the CNS.” Aleta’s CAR T cell engagers provide a way of targeting the HER2 positive cancer cells by ‘coating’ the HER2 protein with the CD19 protein. That way, anti-CD19 CAR T cells are able to engage with the HER2 positive cancer cells and kill them.
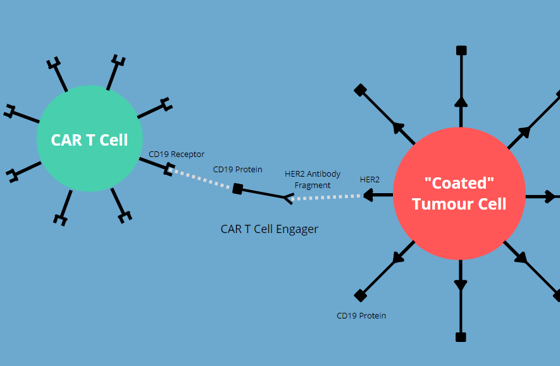
Infographic: CAR T engagers “coat” tumour cells, making them susceptible to CAR T cells
Cleverly, Aleta has encoded their CAR T cell engager into CAR T cells. Therefore, those cells will secrete their own engagers extracellularly to coat the target tumour cell. This gets around the problem of crossing the blood–brain barrier, as anti-CD19 CAR T cells are easily trafficked into the CNS.
“So, our hope is, by marrying the expansion and persistence properties of the CAR to its trafficking potential and its multi-antigen targeting, we can provide a solution for that specific indication,” said Rennert.
Combination Therapy to Achieve Multi-Modal Efficacy
Eduardo Domínguez Medina is a Senior Investigator at the Center for Research in Molecular Medicine and Chronic Disease at the University of Santiago de Compostela. Our panel moderator, Vineeta Tripathi, Chief Executive Officer, and Chief Scientific Officer of Vitarka, asked Domínguez for his thoughts regarding combination therapy as a method of achieving multi-modal efficacy.
Domínguez commented that therapeutic modalities are often based on the evidence we have to hand for the patient population. He continued, explaining that often a patient with cancer is treated by a plurality of therapeutics simultaneously. These could be, says Domínguez, “chemotherapy, targeted therapies, and probably immunotherapies. Still, the efficacy and safety are not good enough to stop the progression of the disease.”
According to Domínguez, this is due to scientists still encountering the challenge of understanding how a lung, breast or colorectal cancer tumour grows and then metastasises in certain patient populations. “I think, perhaps we need to look harder at companion diagnostics and biomarkers as a way to stratify patients. Perhaps only looking at the tumour is not enough, we also need to consider its microenvironment,” he suggests.
Domínguez is hopeful that new technologies will allow for better optimisation of the tools that researchers already have, what he sees as “the next generation of combination therapies.” Tripathi strongly agreed with this point, stating that companion diagnostic and biomarker development were “equally critical” as developing new therapies.
This was a point that Peter Ellmark, Chief Scientific Officer of Alligator Bioscience, chimed in on. Ellmark commented that it was essential for patients that don’t respond to their treatment that researchers investigate potential combination therapies to address their key needs.
Ellmark is “absolutely convinced” that combination therapies will be necessary as well as new and better compounds. The challenge here is that all possible combination therapies cannot be trialled in the clinic, Ellmark asserted. He then stressed the importance of understanding all the data that is being generated, “and improve the understanding of the tumour microenvironment to guide us to which combination would work in which patient population or sub-population.”
Gain valuable insights into the approaches impacting the immunotherapy field through 50+ outstanding presentations tackling key discussion points in immuno-oncology, immunology, and inflammation. Join us at our upcoming Immuno UK: In-Person conference.







