First Paediatric Patients Enrolled in Phase III Clinical Trial for Rare Genetic Disease

The first patient has been enrolled in a global phase III clinical trial for treating activated phosphoinositide 3-kinase delta syndrome (APDS) in children. The drug leniolisib is developed by biopharmaceutical company Pharming, and is an oral, selective phosphoinositide 3-kinase delta (PI3K?) inhibitor.
APDS is a rare genetic immunodeficiency disease where gene mutations cause an impaired immune system in patients.
The study will enrol 15 children with APDS between the ages of four and eleven years old and will test the safety, tolerability, and efficacy of leniolisib. Sites in the US, Europe, and Japan will recruit participants for the trial.
- Addressing Specific Targets and Dealing with Target Engagement
- University of Minnesota Awarded $6.5m for Male Contraceptive Discovery Effort
- Improved Insight on the Effects of Ritalin on Patients with ADHD
Pharming said that the primary efficacy endpoints of the study will be a reduction in index lymph node size and an increased ratio of naïve B cells among total B cells after 12 weeks.
Secondary efficacy endpoints will measure patients’ quality of life relating to their health. A patient questionnaire will seek to quantify physical, social, emotional, and educational functioning.
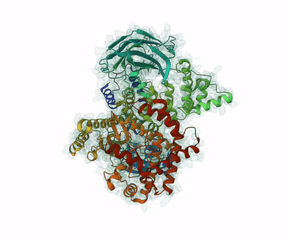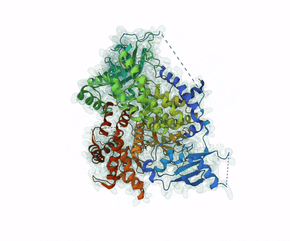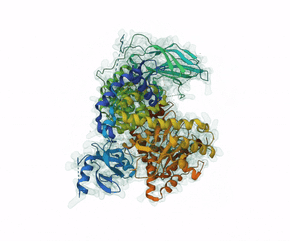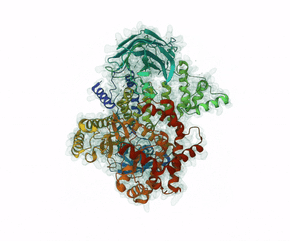
Leniolisib is a small-molecule inhibitor of PI3K?, an enzyme mainly expressed on hematopoietic cells which regulates the adaptive immune system.
The PI3K? signalling helps the maturation of T and B cells. However, in patients with APDS, this signalling is overactive, which can affect T and B cell differentiation. Therefore, the immune system is unable to challenge infections that it encounters.
Currently, the only form of treatment for the disease is to administer antibiotics and antivirals to treat infections.
"In treating APDS, the current standard of care is to use an array of supportive therapies. While these therapies can treat some of the manifestations of APDS, they do not target the underlying cause of the disease,” said Manish Butte, Professor in the Department of Pediatrics at UCLA.
He added: “Pharming's studies of leniolisib in children with APDS are important for evaluating the possibility of minimizing symptoms earlier in the disease progression."
The new clinical trial comes after a successful phase II/III study on leniolisib’s treatment of APDS patients 12 and older. That study concluded that the drug had met both its co-primary endpoints in the older patient population.
A further study by Pharming on the effects of a formulation of leniolisib for even younger paediatric patients (one to six year olds) is expected to run from the third quarter of 2023.
“While continuing to focus on patients of all ages with APDS, we are dedicated to collaborating with regulatory authorities with the goal of generating regulatory filings to gain approval that could support the treatment of children under 12 years of age who are living with this disease," said Anurag Relan, Chief Medical Officer of Pharming.
Get your weekly dose of industry news?here?and keep up to date with the latest?‘Industry Spotlight’ posts.?For other Discovery content, please visit the?Discovery Content Portal.