Digital Twins: Upstream Processing Applications and Hybrid Models

Presented by Joachim Bär, Director of Mammalian Cell Culture Process Development at Boehringer Ingelheim
Transcribed by Ben Norris
Digital twins offer a comprehensive means of making research more efficient and dependable. For Joachim Bär, Director of Mammalian Cell Culture Process Development at Boehringer Ingelheim, they could revolutionise the manufacturing process. As he explained, they will hopefully reduce the number of experiments involved and improve the capture of knowledge in chemical engineering processes.
Contemporary Approaches to Hybrid Models
“Process modelling is such a nice term,” opened Joachim Bär at Oxford Global's Cell UK In-Person event last October. “But let's see what we mean by it.” Bär said that process models are digital representations of either single steps or the complete chain of the manufacturing bio process. They can be used to assess, extrapolate and simulate new process conditions, with model applications developed via in-house hardware. Process knowledge is highly correlated with the number of wet-lab experiments carried out, as Bär explained. “You have to have a very strong correlation between experiments and the knowledge gained.”
"Capturing and conserving process understanding accelerates the learning curve and allows us to learn even more about the process in a shorter timeframe."
Drug substance development runs in parallel with other stages of the laboratory development cycle, predominantly the research and clinical stages. However, the acceleration of clinical projects through more efficient workflows and optimised sequencing methods has brought a new challenge. There is now the task of developing a shorter developmental timeline attaining breakthrough status while still maintaining the ability to yield sufficient process understanding to reduce process risk. “Capturing and conserving process understanding by the models allows us to start with ever-increasing knowledge level into our next process,” continued Bär. “And this accelerates the learning curve and allows us to learn even more about the process in a shorter timeframe.”
Digital Twins: Innovative Modelling Techniques for Secure Research
“What we have developed and what I would like to introduce you to today,” said Bär, “is the digital twin that we have set up with our in-house software.” Digital twins are virtual representations of a discovery process that span its lifecycle and update from real-time data to assist in decision-making. They provide holistic process models to break the correlations between wet-lab experiments and process knowledge. “This accelerates the learning curve and allows us to learn even more about the process in a shorter timeframe,” continued Bär.
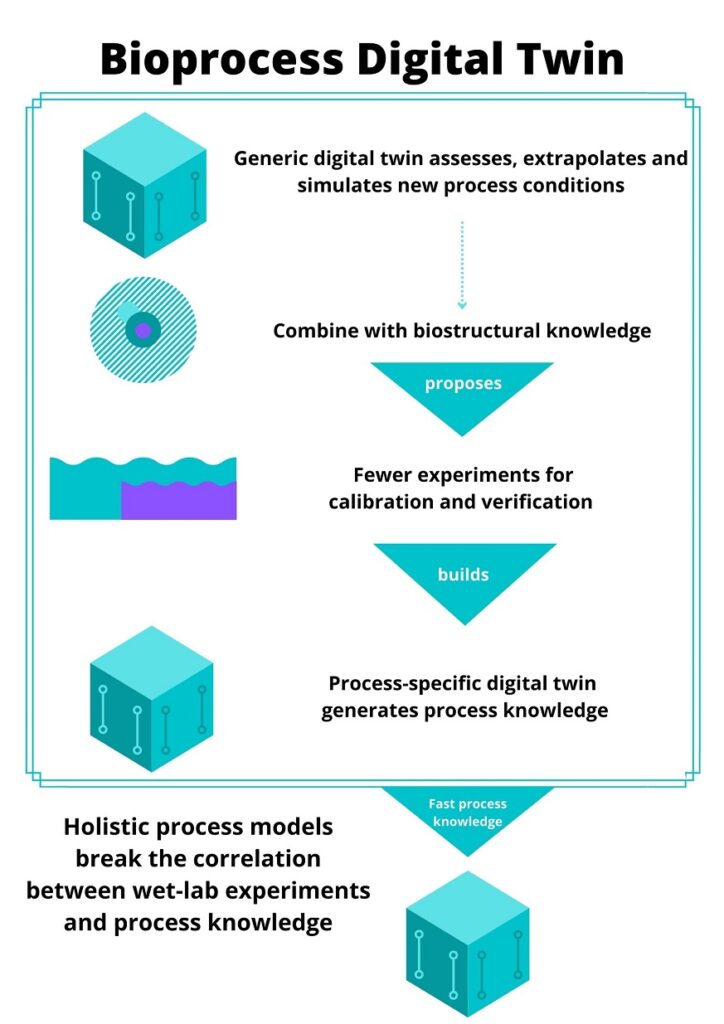
Digital twins as models provide a means of viewing the status of the physical object being investigated. They are encoded with prior knowledge, and their purpose is to reduce the number of experiments being carried out by improving model reliability. As explained by Bär, the current approach is based on the quantity and execution of the experiments performed, with work carried out in critical path development enabling a much faster time to market.
- Understanding Disease Progression with 3D Model Development
- Discussion Group: Future Directions of 3D Model Development for Cell and Gene Therapy
- Tackling Tumours In-Vitro with 3D Cell Cultures
With the knowledge boost associated with new research and development, the process timeline can be reduced to under three years. The capture of knowledge into new models enables risk mitigation, which is central to commercial strategy. One case study provided by Bär came from the microbial world, where data-driven machine learning or artificial intelligence networks are applied to accelerate research processes.
Modelling Approaches with Boehringer Ingelheim Engineering Practices
“Let's shed some light on engineering practices,” continued Bär. Boehringer Ingelheim have a lot of experts coming from different areas, often from backgrounds with different programming languages. “We had to break this Tower of Babel challenge,” said Bär, “and do it in a user-friendly fashion.” With a broad IT and Engineering backbone, setting up a user-friendly front end with a back end available to host various process models is often the best approach. A software platform was developed to easily leverage the full power of integrated process models to rapidly create the best biopharmaceutical manufacturing processes.
"It's one thing to control your outputs, but in addition you should take into account your restrictions."
Some of the processes involved with the digital twin platform include process model repository, process optimisation, process simulation and process robustness assessment. Results from preliminary experiments indicated a substantial productivity increase through machine learning and integrated process modelling. There was a demonstrative >10% titre increase in single unit operation, while Boehringer's Integrated Process Model was capable of optimising multiple unit operations simultaneously. “By interconnecting these three steps, we found a way to optimise our capacities and raise our output by 50%,” continued Bär. “It's one thing to control your inputs, but in addition you should take into account your restrictions.”
Digital Twins and Hybrid Models
The hybrid model includes approaches for mammalian applications, which Bär summarised as 'one platform, one aim'. “It's one thing to control and understand your inputs and how you could define their better process setting,” he said. “But in addition, it's also important that you take into account what your restrictions are.” He added that he was confident there would not be a single model that would capture the entire production process. Instead, it depends on how much data is involved and the individual aims of a particular project. And with this various mathematical model types have to contribute to cover the complete process development and manufacturing steps and phases.
“Hopefully,” Bär concluded, “I could convince you that we have a very nice hosting platform.” Boehringer's in-house process modelling hosting platform is applied to various use cases, with demonstrative applicability for various model formats, be they microbial or mammalian. Additionally, there is upstream and downstream potential on top of different modelling techniques and their hybridization. “We can apply this in development to reduce the effort and time that we need to bring our processes to the market, and also the process for our customers.” The use of process models and digital twins for process development and commercial optimisation suggests untapped potential in two key areas: the reduction of timelines and the subsequent increase of productivity and profitability.
Visit our Cell Portal to find out more about new and current research methods for optimising laboratory modelling. If you'd like to register your interest in our upcoming Gene Therapy Development and Manufacturing In-Person Event, click here.







