GHR Foundation Commits $15m to C2N Diagnostics for Tau Tangle Pathology Test for Diagnosing, Staging Alzheimer’s Disease

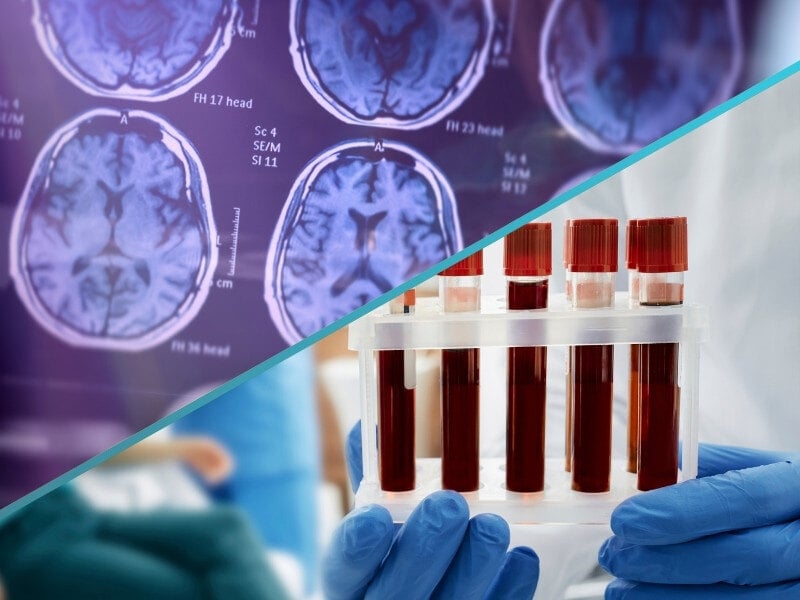
C2N Diagnostics has received a $15 million investment from GHR Foundation to advance its development of a tau tangle pathology test for diagnosing and staging Alzheimer's disease.
This new test will use blood biomarkers, including MTBR-tau, to detect tau tangles, key indicators of Alzheimer's. The test aims to offer a less invasive and more accessible alternative to current diagnostic methods, such as tau PET tracers.
Read more:
- Blood Test for Alzheimer's Granted Breakthrough Device Status by FDA
- Speech-Analysis Tool for the Early Detection of Alzheimer's Handed to Linus Health in Acquisition
- New MND Early Diagnostic Test: a 'Game-Changer' for Research on the Disease
Blood biomarkers like MTBR-tau indicate of pathologies that are associated with Alzheimer’s disease, like tau tangles and neurites. They offer a toolkit for detecting the disease in people with developing cognitive symptoms as well as being influential in the development of the next generation of tau-directed therapies.
“Our innovative, precision medicine-focused work has already galvanized the science, the research, and the platform for developing and commercializing novel diagnostics. Now, we’re poised to add tests for tau-based blood biomarkers to our Alzheimer’s disease testing portfolio,” said Joel Braunstein, CEO at C2N.
“The real benefit of this C2N-GHR collaboration will be seen by patients, families, and health systems currently impacted by the wrath of Alzheimer’s disease.”
This funding builds on GHR’s total $50 million commitment to C2N, supporting innovations that improve Alzheimer’s diagnostics and patient care. Only in September 2024 did GHR pledge $7.025 million from the Alzheimer’s Drug Discovery Foundation’s (ADDF) Diagnostics Accelerator initiative to C2N.





_1.jpg)