Nexelis’s Collaborative Development for Optimal Clinical Assay
Nexelis, a Q2 Solutions Company, has been a market leader in the field of vaccine immunology since its inception as NEOMED-LABS, a spin-off of GSK in 2015. They have supported the development of prophylactic vaccines for over 50 infectious disease targets. In their webinar, hosted by Oxford Global in November 2022, Nexelis showcased their immuno-tools and immuno-assay development.
Nexelis’s protein science team works in close collaboration with their clinical team across the full development cycle. The message of the presentation demonstrated that optimal antigen and reagent characterisation and assay development is a key success to robust and reliable assays.
Main Challenge for the Development of New Assays in Vaccines
Steven Tran, Director, R&D Assay Development, said that the main challenges that face the development of new assays in vaccines was “access to purified and stable antigens, viral-like particles (VLPs), or pseudoviruses.” Moreover, assay controls, standard and sample panel materials need to be delivered in an appropriate timeline.
Tran went on to describe the traditional process of assay development, starting with access to the antigen. For this, assay development requires an antigen provider. “If you are lucky,” said Tran, “a provider may have a few micrograms available.” But Tran added that if a provider does not have the needed antigen in stock, it could incur four to eight weeks of delays.
An added complication of this is the fact that in the traditional assay development process, the quality and purity of the protein will only be known when the recipient obtains and tests the antigens. Tran explained that “if the quality is insufficient, then you have to troubleshoot with the provider and wait for a new batch.”
In order to advance this process, Nexelis have an in-house protein science team that produce the antigens necessary for assay development, validation, and clinical testing. Tran’s team works closely with the protein science team to produce critical reagents and troubleshoot the process of purification and production. “That way we can rapidly have the antigen ready for the assay development to start,” Tran continued.
Nexelis's Project Preparation and Initiation
To make sure that what the sponsor needs is aligned with what is included in the contract, Nexelis will provide an early consultation with their sponsors before a project’s initiation. This discussion will produce a development and validation plan based on the sponsor’s requirements.
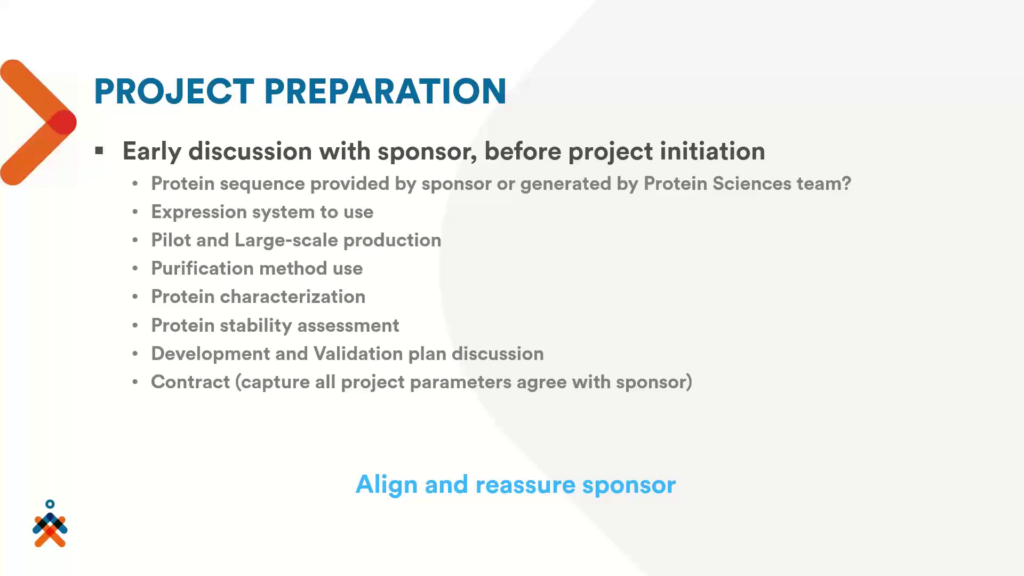
“Once we have captured all of this information, we put all of this on paper so that all the project’s parameters agree with the sponsor,” explained Tran.
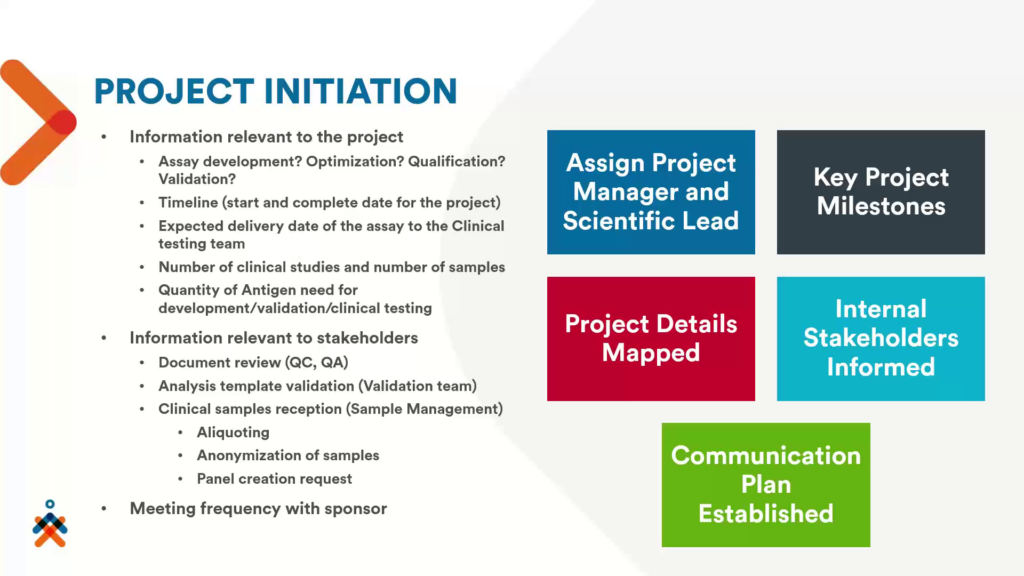
Once the contract is signed, Nexelis have an initiation meeting, inviting all the teams that are involved with the project such as the project manager, R&D and clinical manager, QA/QC, validation team, sample management team, and the protein science team. This meeting is designed to finalise the information relevant to the project, and relevant to the stakeholders (see figure 2).
Protein Science for Producing Recombinant Proteins
Next, Martine Boyer spoke about the protein science team, Boyer is Director of Protein Science at Nexelis. She said that the main goal of her team was to support clinical testing for drug and vaccine development. Herein, the protein science team generate the tools and immunological reagents required for the development of testing assays that support vaccine development.
“Having a team capable of providing custom critical reagents allows Nexelis to offer assays perfectly adapted to the needs of each client and suitable for clinical studies,” explained Boyer. She continued: “in addition to that, it allows access to products which are not available on the market.”
By working closely with the clinical team, the reagents that Nexelis provide are always suited to the needs of the client and are fully characterised. Boyer explained that the key parameters are monitored over time to detect any change that may occur during use. “The advantages of this close collaboration are the reduction in time and cost for clinical studies,” said Boyer.
“The advantages of this close collaboration are the reduction in time and cost for clinical studies”
In the process of recombinant protein production, the protein of interest is inserted into a host cell using a vector to generate that particular protein. Boyer explained that in their case, for clinical assay, this is done in five main steps:
1. The best recombinant protein expression system is selected in order to ensure that that high-quality recombinant proteins are produced. Boyer went over the different expression systems available and their respective advantages and disadvantages.
| Expression System | Advantages | Disadvantages |
|---|---|---|
| E. Coli | Low cost. Rapid expression. High yield. Easy to scale up. | Rare PTM. Inclusion bodies. High MW protein limitation. |
| Yeast | Low cost. Rapid expression. Easy to scale-up. | Non-human-derived glycosylation (high mannose content). |
| Insect Cells | Large gene capacity. Simple PTM. Soluble proteins. High cell density. | Partial glycosylation. Long culture period. High cost. |
| HEK293/CH | Desirable PTM. Soluble proteins. Low endotoxins. Better activity. | High cost. Long culture period. |
“The selection of the appropriate expression system will depend on different variables, and also in the need for downstream application,” said Boyer. These factors include structural complexity, solubility, cellular localisation, post-translational modifications, and investment.
2. Next, the most optimal construct is determined whereby the coding sequence is within active parameters in the chosen expression system. Here, Boyer’s team analyse the desired gene sequence, mainly for transmembrane or disordered regions, and for optimisation of the signal sequence.
Another part of the cloning strategy is to determine the necessity of the coding sequence’s expression in-frame with an affinity tag. Finally, to increase the production yield, the sequence of the codon has to be optimised for the expression system selected. After confirmation of the sequence, the DNA is produced for expression.
3. The protein science team next perform expression optimisation in small-scale by screening various conditions in parallel. Then, Nexelis transition to large-scale production using the best conditions and most suitable expression system for the protein of interest. “Not all expression systems are equal,” said Boyer. “And sometimes performing comparative evaluation of different constructs in different small-scale expression systems can be very helpful,” she added.
The optimisation process consists of monitoring the cells daily for the best expression conditions, evaluating the growth duration and temperature. “Parallel screening of constructs and identification of the key parameters, quickly leads to the identification of the optimal constructs and the best expression system,” explained Boyer.
Before they scale up the production of the protein, Martine’s team perform a pilot study to determine the yield. In conjunction with Steven Tran’s team, they perform an immunoassay evaluation to ensure that all the parameters are adequate before scaling up production.
4. Nexelis then produce the protein purification strategy using different combinations of chromatography, “the objective is to reach the highest level of purification in the fewest steps,” said Boyer. “Nevertheless, it is inevitable that each step of protein purification will result in some amount of protein loss,” she added.
The most widely used method for the purification step is affinity chromatography, which “separates proteins based on their interaction with the matrix.” Boyer explained that they use different chromatography techniques in combination at this stage in the process in order to reach the required purity for use in clinical assays.
5. The final checkpoint of any protein production process is to evaluate the critical attribute before downstream application. “Ignoring this step can result in irreproducible and misleading observations in downstream application,” warned Boyer. Nexelis’s approach here uses different physio-chemical technologies to evaluate essential properties of any protein sample. According to Boyer, the purpose is to “provide clear information about the size, shape, purity, aggregation state, and behaviour of the purified protein.”
“The assessment and optimisation of the quality of the protein significantly increases the chance of success in downstream experiments,” Boyer added. The goal is the generation of quality reagents and lot-to-lot reproducibility.
[elementor-template id="29448"]







