Translational Biomarkers to Guide Clinical Development of CAR T-Cells

Our March group came together for an hour of specialist discussion about the current state of CAR T-Cell therapies for biomarker development. This Discussion Group consisted of a select group of industry leaders from various pharmaceutical and biotech companies. Kathryn Newhall, VP of Translational Clinical Development at Cellectis, and John Rossi, Head of Translational Medicine and SVP of Research at CERo Therapeutics, lead the session.
The discussion covered key topics from CAR T development, tumour clearance programs, and optimal knockout approaches. Notable attendees included senior representatives from AbbVie, Bayer, Catalyst Biosciences, Sanofi, Intelligent Omics Ltd, and EPO Berlin-Buch GmbH, to name a few.
Chimeric Engulfment Receptor T-Cells:
Rossi kicked off the discussion with a presentation on the ‘Development and Functional Characterisation of Chimeric Engulfment Receptor (CER) T-Cells.’ During this, Rossi reviewed what he proposed to be the key learning takeaways from working with CAR T-Cells. Rossi opened the presentation with a brief development timeline, explaining how the first T-Cell receptor therapy, Kimmtrak, was approved in January 2022 for uveal melanoma.
"Solid tumour indications remain the largest and most current unmet need in oncology".
The use of Chimeric Antigen Receptor (CAR) as a biomarker has now been approved for various NHL indications in Japan, China, Canada, Switzerland, Israel, and Australia. However, solid tumour indications remain the largest and most current unmet need in oncology. Other concerns include the lengthy approval time for products to reach commercial viability, which currently stands at approximately 30 years.
Speaking about research conducted whilst previously serving as Senior Director at Kite Pharma, Rossi divulged findings concerning the durability of response for CAR T-Cells. In a pivotal ZUMA-1 study, data showed that early expansion was the key driver of response. “There was a rapid expansion of CAR T-Cells in patients within the first month after infusion, particularly within the first 7 to 14 days and then a persistence over time,” Rossi explained (see figure. 1).
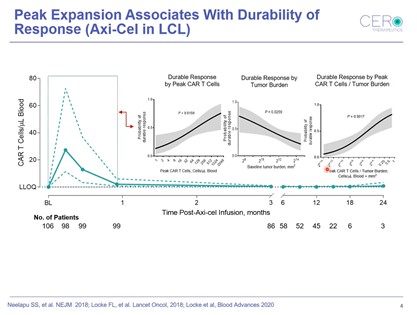
The higher the peak expansion, the higher the probability of a patient remaining in response. “One of the things we also showed early on at Kite was relativity to tumour burden, when probability of a durable response decreases,” Rossi added.
Other key takeaways include understanding the factors affecting PK, such as doubling time. Doubling time is a metric used to measure how quickly a T-Cell divides relative to the viability of the cells over the course of manufacturing. Kite Pharma discovered that T-Cell fitness and the presence of cell subsets drives expansion. Rossi summarised the findings as “the more juvenile the T-Cell subset in the pre-infusion product, the faster the doubling time during manufacturing, and the better the PK profile of those patients.”
The CER T Cell Platform Technology:
Rossi then presented CERo’s CER T-Cell platform technology which represents a novel class of engineered T-cells that converges innate and adaptive tumour clearance programs into a single T-Cell. As a unique multimodal mechanism of action, the platform provides recognition of a tumour agnostic and inducible target as well as identification of engulfed tumour fragments.
The technology also enhances APC-like function, induces cytotoxicity, and acts synergistically with targeted agents. So far, CERo has found target-specific cell killing demonstrated in both hematologic and solid tumours.
Allogeneic CAR T-Cell Therapies:
Newhall then delivered a presentation entitled ‘Translational Biomarkers to Guide Clinical Development for Allogeneic CAR T-Cell Therapies.’ In this, Newhall discussed some of Cellectis’ clinical and translational data. Highlighting the benefits of CAR T-Cell therapies, the presentation touched on the possibilities of scalable manufacturing, off-the-shelf availability, efficiency and robustness, and market access.
Newhall explained her role as being “involved in escalation studies such as monitoring biomarkers and pharmacodynamic response to identify the optimal dose of our products.” She then delved into the biotech’s use of its pioneering gene-editing platform to develop life-saving cell and gene therapies. Currently, Cellectis has three clinical stage CAR T-Cell product candidates: CD123 for acute myeloid leukaemia, CD22 for acute lymphoblastic leukaemia, and CS1/SLAMF7 for multiple myeloma (see figure. 2).
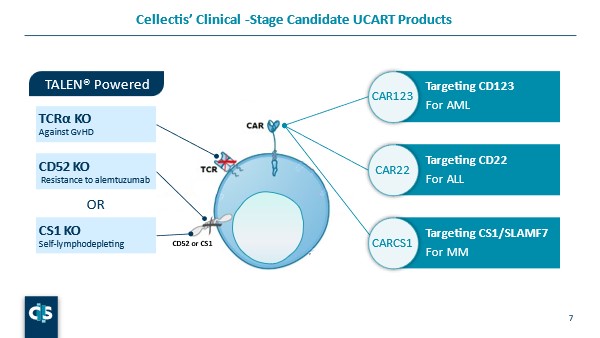
“All of these therapies have a knockout the constant region of TCRα chain of the T-cell receptor alpha constant (TRAC) gene prevent graft versus host disease or GVHD,” Newhall explained. UCART123 and UCART22 also have a CD52 KO “so that we can utilise anti-CD52 antibodies such as alemtuzumab to lymphodeplete the endogenous immune cells,” she continued. CS1 is also expressed on endogenous immune cells, and UCARTCS1 contains a CS1 knockout to avoid self killing of the CAR T-cells.
Cellectis has administered over 170 patients with their allogenic technology. The studies have demonstrated safe and effective engraftment and redosing feasibility. Newhall concluded that TCRa knockout results in safe non-alloreactive UCART, and that anti-tumour activity was shown to be consistent with autologous products. “In terms of overall safety, our data showed that our products are on par with approved autologous CAR T-cell therapies, which is very promising,” Newhall added.
Q & A: Translation to the Clinic
After the introductory presentations concluded, the leaders opened the floor up for questions. One audience member asked whether either leader observed phagocytosis in their experiments, and if so whether this could be a result of an autologous setting.
- How has the Pandemic Revolutionised Clinical Trials?
- How can the Industry Streamline Clinical Testing in Precision Medicine?
- What are the Challenges of Clinical Biomarkers for Neurological Disorders and Oncology?
Rossi responded that “at CEROs we have tried to observe this the best we can when we have a close HLA match or an uptake of a pHrodo labelled cell line, as well as localisation to the lysosome.” He continued, “the phagocyte receptor seems to be agnostic to whether or not there is a match to an allogeneic approach.” Newhall concurred, attesting to the value of such a determination but acknowledging that it is not yet well defined.
A further question posed inquired about the use of patient-derived models to identify potential responders and non-responders. Newhall acknowledged this approach as an optimal strategy to address translational hurdles such as variability. However, a considerable challenge of patient-derived models is being able to access enough available participants.
“In terms of overall safety, our data showed that our products are on par with approved autologous CAR T-cell therapies, which is very promising,” Newhall added.
The discussion group concluded with some final thoughts on the future of CAR T-Cell therapies. With ongoing research and development in CAR T engineering, the future looks bright for this particular field of biomarkers. We couldn’t have been more pleased with the turnout for our March biomarkers Discussion Group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our discussion group series in April with a session focusing on Fluid Biomarkers for Neurodegenerative Disease. Learn more about the Oxford Global discussion group series at our Biomarker Portal.
Want to stay up to date with the latest Biomarker news? Register now for Oxford Global’s flagship event, Biomarkers US: In-Person. This is a must-attend forum covering the latest trends transforming biomarker and translational research.





.jpg)
