Microfluidic Droplet-Based PCR: a Tool to Solve Challenges in Personalised Medicine and Cancer Detection?
Presented by: Dr Valerie Taly, Group Leader & CNRS Research Director, Cordeliers Research Center, INSERM, University Paris Cité
Transcribed by: Ben Norris
Dr Valerie Taly, Group Leader & CNRS Research Director, gave an excellent speech addressing the potential applications which are leading research in droplet-based digital PCR for cancer patients. This article will further explore the applications of ddPCR in the early detection of mutated or methylated tumour circulating DNA in cancer patients and what this could mean for future screening through liquid biopsy.
Droplet-Based PCR and Mutation Detections Through Liquid Biopsy
Although DNA sequencing techniques have come on in leaps and bounds since their inception in the 1970s, significant challenges remain in the blanket detection of localised and metastatic colorectal cancers. Headed by Dr Taly, the Translational Research and Microfluidics (TRAM) Research Group at the University of Paris Cité had identified an avenue for applying quantitative approaches to biomarker detection, which could potentially increase the reliability of cancer screening.
As she explained at Oxford Global’s 2021 Next-Gen Omics: In-Person UK event, the approach worked on by Dr Taly’s team involves looking for genetic or epigenetic alterations associated with the development of cancer, which serve as acutely specific biomarkers. One of the primary methods for this is liquid biopsy, where fluid samples are investigated for tumour related elements (including nucleic acids or cells) that may be circulating in the blood.
Specific DNA sequences are commonly detected using conventional polymerase chain reaction (PCR); improvements in detection methods such as droplet-based digital PCR (ddPCR) for detecting rare events like the early onset of cancers have enabled their use in clinical settings. The prompt detection of cancers through circulating tumour DNA (ctDNA) analysis could have a significant impact on the morbidity and mortality of localised and metastatic colorectal cancer patients.
Complications of Scale Hindering PCR Analysis
However, bloodborne ctDNA concentrations are often fractional relative to concentrations of normal DNA coming from non-tumour cells. “Here comes the need for sensitivity,” Dr Taly explained. “You’re really at the single-molecule level – ctDNA can be detected within blood samples, but since it presides in very low amounts it may only represent a tiny fraction of the total circulating DNA.” Conventional PCR-based procedures presented significant limitations to research when the project began, back in 2008, while the highly sensitive procedures necessary to detect trace ctDNA cannot do so at very low quantities.
“Here comes the need for sensitivity… ctDNA can be detected in blood samples, but since it presides in very low amounts it may only represent a tiny fraction of the total circulating DNA.”
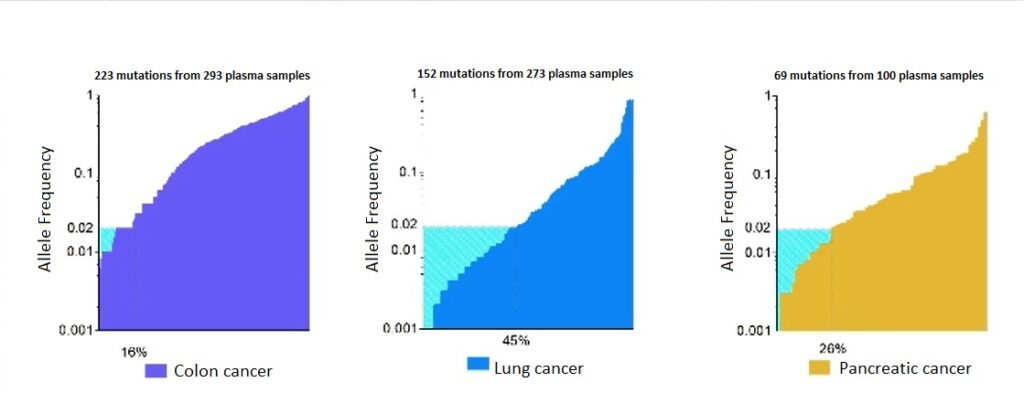
Data collected by the research team on colorectal cancer, lung cancer and pancreatic cancer suggested the typical concentrations of ctDNA that may be anticipated in patients with advanced cases of these cancers. “We looked into how many patients will have less than 2% of ctDNA, and this fraction is quite high,” continued Dr Taly. “It’s 16% in colon cancer, 45% in lung cancer and 26% in pancreatic cancer.” Essentially, a high proportion of patients will demonstrate a low amount of ctDNA even in cases of advanced cancer (as seen in Figure 1), so the challenge for Dr Taly and her team was the development of a reliable method for ctDNA screening that detected as many mutations as possible.
Applications of ddPCR for Localised and Metastatic Colorectal and Cancer Patients
The solution to the TRAM Research Group’s Gordian Knot concerned the use of digital droplet PCR (ddPCR) to enable the high-resolution identification of mutated DNA, as well as the determination of mutant allelic fractions (mA%). The process involves water in oil droplet technology: a standard sample is fractionated into five to ten million droplets, with PCR amplification of the template molecules occurring in each individual droplet. This approach enabled a far higher degree of accuracy across a much greater sample size than previous studies, circumventing the obstacle of high-resolution sampling which slowed down conventional PCR techniques.
Dr Taly’s laboratory assembled what she affectionately described as “homemade microfluidic stations” for development and platform design. Dedicated improvements included a customised optical setup and microfluidic chips; a clinical analysis of samples was carried out in collaboration with Raindance Technologies, using the company’s RainDrop system for assay development and clinical validation. Screening for universal colorectal cancer markers was also assessed in droplets with the use of methylated markers, enabling researchers to discriminate malign tumour tissues from healthy adjacent tissues. An analysis of 56 tumour tissues revealed that all were positive, with 94.3% positive for one marker.
- 📹 Watch: Exclusive Interview with Ed Schuuring of University Medical Center Groningen About the Future of dPCR
- Discover: Potential Applications of Liquid Biopsy for Easy and Difficult Cancers
- Read: NextGen Omics Report on Current Market Trends and Industry Challenges
Dr Taly told the audience that ddPCR allowed for the performance of quantitative and highly sensitive measurements at a resolution of 1/200,000 mutant DNA in wild-type DNA, as opposed to 1/10 in bulk experiments. Even at high dilutions, the detection of target sequences through this procedure is quantitative, making it an efficient means for identifying mutant sequences and a potential tool for addressing challenges in personalised medicine. Highly sensitive methods are required for ctDNA detection and quantification in order to detect cancerous or otherwise malign DNA. Even advanced cancer patients often registered less than 2% ctDNA in their plasma; earlier cancer developmental stages aligned with the lower patient detection through ctDNA monitoring.
Future Applications of Liquid Biopsy for Cancer Detection
The main conclusions from this research were that a ctDNA follow-up could be a pertinent marker of cancer progression, since diagnostics suggest a correlation with conventional markers. Moreover, a ctDNA follow-up could be the only molecular marker of cancer progression for CEA-negative patients. One of the many practical applications of this procedure is that healthcare providers could determine if a particular chemotherapy treatment is effective after one cycle, instead of administering several rounds of unsuccessful therapy. Dr Taly concluded her presentation by asserting that liquid biopsy could be used to track tumour DNA in advanced and early colorectal cancer patients, with optimism for its use in clinical settings in years to come.
Dr Taly will be speaking with Oxford Global again at this year's NextGen Omics UK event, running from November 09 to November 10. To register your interest or find out more, visit the NextGen Omics portal.
References
Postel, M., Roosen, A., Laurent-Puig, P., Taly, V. and Wang-Renault, S., 2017. Droplet-based digital PCR and next generation sequencing for monitoring circulating tumor DNA: a cancer diagnostic perspective. Expert Review of Molecular Diagnostics, 18(1), pp.7-17.






