Fluid Biomarkers for Neurodegenerative Disease

Our April group came together for an hour of specialist discussion about the current state and future directions of novel fluid biomarkers for neurodegenerative disease development and diagnosis. This Discussion Group was a select group of key industry leaders from various pharmaceutical companies and research institutions.
Jordi Clarimon, Lead Specialist in Fluid Biomarkers at Lundbeck led the discussion, covering key topics from clinical utility for biomarkers and overcoming the challenges of new methodological approaches, to technological advances. Notable industry attendees included senior representatives from INmune Bio, AbbVie, Hoffmann-La Roche Ltd., and Takeda. Academic attendees were Professors from the University of Oxford and the University of New Mexico, to name a few.
The Purpose of Biomarkers in Clinical Trials:
Clarimon kicked started the session by comparing regulatory definitions of a biomarker. The EMA definition reads: “Biological molecule found in blood, other body fluids, or tissues that can be used to follow body processes and diseases in humans and animals.” The FDA on the other hand, identifies biomarkers as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention, including therapeutic interventions.”
Contrasting biomarker qualification highlights the regulatory landscape’s dynamic and sometimes contradictory nature. As Clarimon puts it, “it is interesting to differentiate between the two definitions because it attests to the broad scope of the biomarker field.” Clarimon went on to explain the purpose and utility of biomarkers in a clinical trial setting.
Contrasting biomarker qualification highlights the regulatory landscape’s dynamic and sometimes contradictory nature.
Biomarkers are clinical tools that detect a signal to support internal decision making and the development plan. They can de-risk the clinical development process and ideally provide surrogate endpoints to facilitate accelerated approvals in both common and rare diseases. ADUHELM is an example of a surrogate biomarker that reduces amyloid beta plaques to predict the clinical benefit for Alzheimer’s patients. Although controversial, the FDA approval was based on a surrogate biomarker readout.
Reproducibility Issues in Biomarkers for Neurodegenerative Disorders:
“Context of use is key for a biomarker of utility,” Clarimon continued. Fluid biomarkers for treatment of neurodegenerative disease is an area of much promise. Primary fluid biomarkers include Abeta (Aβ42), P-tau, NFL, neurogranin, GFAP etc. (see figure.1 for extensive list). However, few fluid biomarkers have been robustly associated with neurodegenerative diseases, and more work into other, non-AD neurodegenerative disorders, is needed.

Figure. 1 – A list of fluid biomarkers in neurodegenerative disease as referenced in Hansson O. Nat Med 2021
Many have undergone several attempts of replication or have shown conflicting results. “There is also a lack of consensus on how to optimally use fluid biomarkers for Alzheimer’s in practice,” Clarimon claimed. “Although there is a statistically significant difference in biomarkers used for research studies,” he added, “the degree of change can often be too low to fill the demands for a useful diagnostic test in the clinic.” As such, most biomarkers have failed replication, especially in omics and panel studies.
Optimal Matrices for Amyloid Pathology:
According to Clarimon, “abeta in plasma is promising yet undeveloped for Alzheimer’s disease.” This is because its predictive accuracy differs between assays, and there is a large overlap in plasma (see figure. 2). Nevertheless, using fully automated platforms to certify reference material can work towards overcoming these challenges. In particular, the Global Consortium for the Standardization of CSF Biomarkers has introduced an automated technology to provide low analytical variability and low lot-to-lot-difference.
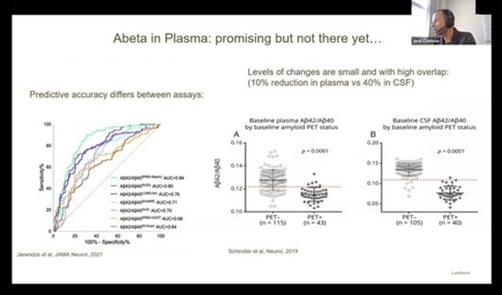
Figure. 2 – Graphs displaying the predictive accuracy and overlap for Abeta in Plasma
Other plasma-based biomarkers include tau, a microtubule-associated protein that is abundant in the neuron's axons, and a variety of phosphorylated isoforms. Tau stabilises microtubule bundles and plays a central role in regulating microtubule assembly, dynamic behavioural output, and spatial organisation accent.
The evident relationship between tau aggregation and cognitive symptoms of Alzheimer’s disease continuum has led to identification of tau-related therapeutic targets. These targets are microtubule stabilising agents, modulation of autophagy or proteasomal digestion, and aggregation and expression inhibitors. “Using these fluid biomarkers, Alzeihemer’s can be diagnosed before the dementia stage,” Clarimon indicated.
New Technologies:
Advanced technologies aim to provide a more sensitive and accessible form of self-monitoring for fluid biomarkers in neurological diseases. Clarimon predicts that new technologies will be cheaper and “provides less sample volume, which is important for the CSF.” Monitoring equipment will also see a faster and more efficient turnaround time.
- Cultivating Neuro-Organoids for Translational Research
- What are the Challenges of Clinical Biomarkers for Neurological Disorders and Oncology?
- Using Human Stem Cell Models for Neurodegenerative Diseases
“Companies are trying to make these new and easy-to-work tools to conduct reliable and easy monitoring,” he explained. In particular, Aligned Bio has developed a single-molecule detection system with light-guiding nanowires to determine protein concentrations and diffusivity. Other advances include MagQu’s Immunomagnetic Resolution technology which harnesses magnetic particle rotation to generate signals. Clarimon concluded that “the numerous advances in computerised measurement on the horizon promises to lead to a more robust set of biomarkers in the clinical setting.” Also, the improvement of some well-established assays such as the Seeding Amplification Asssay, could allow a more quantitative or semi-quantitative measurements, which would be very beneficial in clinical trials.
Final Remarks:
In response to the presentation, audience members inquired about Clarimon’s thoughts on the possibility of using exosomes for biomarker development. Clarimon mentioned how incorporating Extracellular Vesicles (EVs) into biomarker design can provide precision diagnostics, although many unsolved problems (e.g. no standardised protocols for isolation or characterisation) can give rise to an extra layer of complexity to interpret the outcomes. Likewise, one audience member acknowledged that “exosomes more accurately reflect brain expression levels, offering insight into the mechanistic aspect of neurodegenerative disease development.”
Advanced technologies aim to provide a more sensitive and accessible form of self-monitoring for fluid biomarkers in neurological diseases.
The discussion concluded with some final thoughts on the potential of fluid biomarkers for neurodegenerative disease. At Oxford Global, we couldn’t have been more pleased with the turnout for our April biomarkers Discussion Group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our Discussion Group series in May with a session focusing on ‘Spatial Biomarkers & Multiplexed Immunofluorescence in Immuno-oncology’. Learn more about the Oxford Global Discussion Group series at our Biomarkers Portal.
Want to stay up to date with the latest Biomarker news? Register now for Oxford Global’s flagship event, Biomarkers US: In-Person. This is a must-attend forum covering the latest trends transforming biomarker and translational research.





.jpg)
