Why We Develop Autoimmunity: Hyperstimulation of Genetically Prone Subjects
Presented by: Yehuda Shoenfeld, Professor at Tel-Aviv University
Edited by: Cara Digby-Patel
The precise cause of autoimmune diseases, which occur when the body’s immune system begins to attack the body’s own healthy tissue, has long been under discussion. Professor Yehuda Shoenfeld, the founder and head of the Zsabludowicz Centre for Autoimmune Diseases at the Sheba Medical Centre, has spent much of his career working to establish precisely how we might understand what causes autoimmunity to arise.
Speaking at Oxford Global’s Biologics UK: In Person 2021 conference, Shoenfeld presented research supporting his theory that autoimmune diseases are more likely to develop in subjects with a genetic predisposition whose immune systems undergo hyperstimulation due to environmental factors.
The Mosaic of Autoimmunity
Shoenfeld’s model for the development of autoimmunity has been refined throughout his research, which spans nearly 30 years. He has established “the mosaic of autoimmunity,” which reflects “the multifactorial origin and the diversity of expression of autoimmune diseases.”
- Widening the Therapeutic Window: Development of Antibody Drug Conjugates for Solid Tumours
- Bispecific CAR-T Cell Therapies: Successful Applications in Mouse Tumour Models
- Sane in the Membrane: Salipro One-Step Reconstitution of GPCRs and Ion Channels
Shoenfeld espoused the view that genetic predisposition is perhaps the most significant determinant of whether an individual will develop an autoimmune disease; in particular, he focuses on the HLA-DRB1 gene, which he deems to be “notorious” for its links to autoimmunity. He cited a 2017 study in particular, which revealed that almost every autoimmune disease is more prevalent amongst those who carry the HLA-DRB1 allele, as it causes a stronger, more aggressive immune system.
When coupled with the hyperstimulation of the individual’s immune system by any environmental factor, this genetic susceptibility will lead to the production of auto-reactive T-cells, and eventually the development of autoimmune diseases.
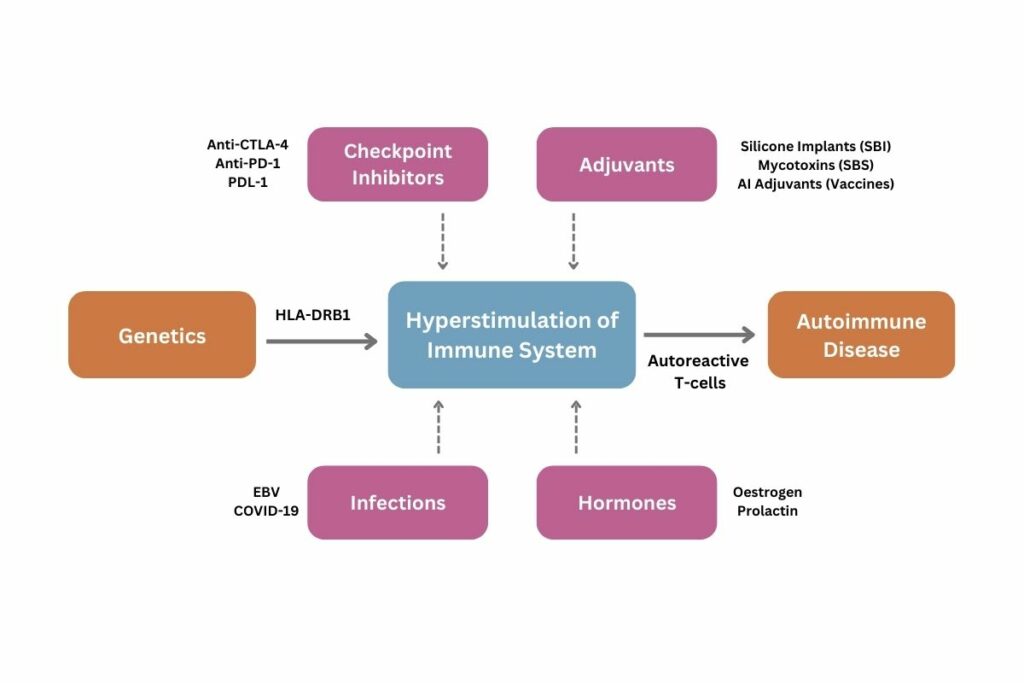
Shoenfeld briefly addressed the associations between external stimuli such as hormones and infections which are widely accepted, with particular reference to the links drawn between COVID-19 infections and the development of certain autoimmune diseases. However, his primary focus was to demonstrate that both checkpoint inhibitors and adjuvants can provoke hyperstimulation, ultimately leading to an autoimmune response.
Checkpoint Inhibitors as Artificial Stimulants
In 2018, Allison and Honjo won the Nobel Prize for discovering a means to overcome incurable cancers via immunotherapy. Fundamentally, they established that if checkpoints which blocked the immune system and prevented its overstimulation were unblocked using checkpoint inhibitors such as anti-CTLA-4, the subsequent hyperstimulation would allow for the immune system to combat previously incurable cancer.
However, the toll of this process is that up to 30% of subjects treated using checkpoint inhibitors developed symptoms of autoimmune diseases, commonly referred to as immune-related adverse events (irAEs) or immune-mediated adverse reactions (IMARs). As Shoenfeld put it, “checkpoint inhibitors have revolutionised the treatment of cancer, but can also cause a spectrum of unique adverse events of autoimmune or autoinflammatory nature.”
Of those cancer patients exhibiting autoimmune symptoms, approximately 20% went on to develop fully-fledged autoimmune diseases. All those who fell within this category possessed the HLA-DRB1 allele. Therefore, Shoenfeld concluded that the hyperstimulation of a genetically prone subject's immune system will lead to the development of autoantibodies and eventually an autoimmune disease.
The Dangers of Silicone Implants
The risks presented by silicone implants are not a recent discovery. For years, patients frequently complained about a diverse array of symptoms, ranging from chronic fatigue and pain to memory loss and even deafness. These symptoms arise when silicone bleeds from the implant and spreads throughout the body. This is because, crucially, silicone is not inert; rather, it is an adjuvant, a substance which enhances the body’s immune response, resulting in the overstimulation of the immune system.
Shoenfeld argues that the impact of silicone and the body’s response is evidence for a syndrome he has proposed called Autoimmune Syndrome Induced by Adjuvants, or ASIA. Shoenfeld characterises this syndrome as a pre-autoimmune disease whose symptoms are not distinctive enough to diagnose a specific autoimmune disease. The major criteria for ASIA include chronic fatigue, persistent pain, and cognitive impairment, all of which were reported by women with silicone implants. One of the most important minor criteria is that the patient will possess a specific HLA gene, such as HLA-DRB1.
The progression from these symptoms into a fully-fledged autoimmune disease can be observed through studies on women with silicone implants. Shoenfeld cited his 2018 study which established that almost all autoimmune diseases are more prevalent in women with these implants. Furthermore, the study found that all the women who ultimately developed autoimmunity had the HLA-DRB1 allele.
Silicone functioning as an adjuvant can therefore be taken as further proof that when an individual with a genetic predisposition is exposed to an environmental factor which will provoke the hyperstimulation of the immune system, they will eventually develop an autoimmune disease.
Shoenfeld concluded by citing the potential for predicting people’s risk of developing an autoimmune disease if their genetic composition is analysed when artificial stimulants such as checkpoint inhibitors or adjuvants are being introduced into an individual’s system.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.









