Viral Immunotherapy: A New Approach Towards Tackling Solid Tumours
Presented by: Paul Peter Tak, President, Chief Executive Officer, and Board Director, Candel Therapeutics
Edited by: Ben Norris
High-grade gliomas are some of the most challenging cancers to treat. Tumours that develop in glial cells surrounding nerve cells are difficult to target through more traditional therapeutic methods, and survivability rates for treatment are typically very low. For Paul Peter Tak, President, Chief Executive Officer, and Board Director at Candel Therapeutics, this is a challenge to confront and overcome. “We want to share the potential, not just of doing great science, but of transforming the lives of patients with cancers that are really difficult to treat,” said Tak. The aim is to treat specific, difficult-to-target tumours with low survivability.
Treatment Overview: CAN-2409 and Viral Immunotherapy
Viral immunotherapeutics are currently being evaluated in patients with high-grade glioma: a kind of tumour that occurs in the brain and spinal cord. Two key investigational medicines are currently being pioneered as potential treatments: CAN-2409 and CAN-3110. As Tak explained to the audience at Immuno UK: 2022, CAN-2409 is an engineered adenovirus-based gene construct which encodes HSV thymidine kinase. Known also as aglatimagene besadenovec, it encodes the thymidine kinase gene derived from the herpes simplex virus and is injected directly into tumour or target tissue. It functions as an viral immunotherapy that induces specific tumour-infiltrating T cells.
There are ongoing clinical trials regarding the application of CAN-2409 in non-small cell lung cancer (NSCLC), high-grade glioma (HGG), pancreatic cancer, and prostate cancer. CAN-3110, meanwhile, is a true oncolytic virus which can replicate specifically in the tumour while sparing healthy tissue. Tak explained that recurrent high-grade glioma represented a challenge for treatment as a completely therapy-resistant disease. “These patients do not only have an awful disease with an already poor prognosis, they’ve also failed neurosurgery, chemotherapy, and radiotherapy,” said Tak. “So if you start to see a response then you know it’s not a placebo effect.”
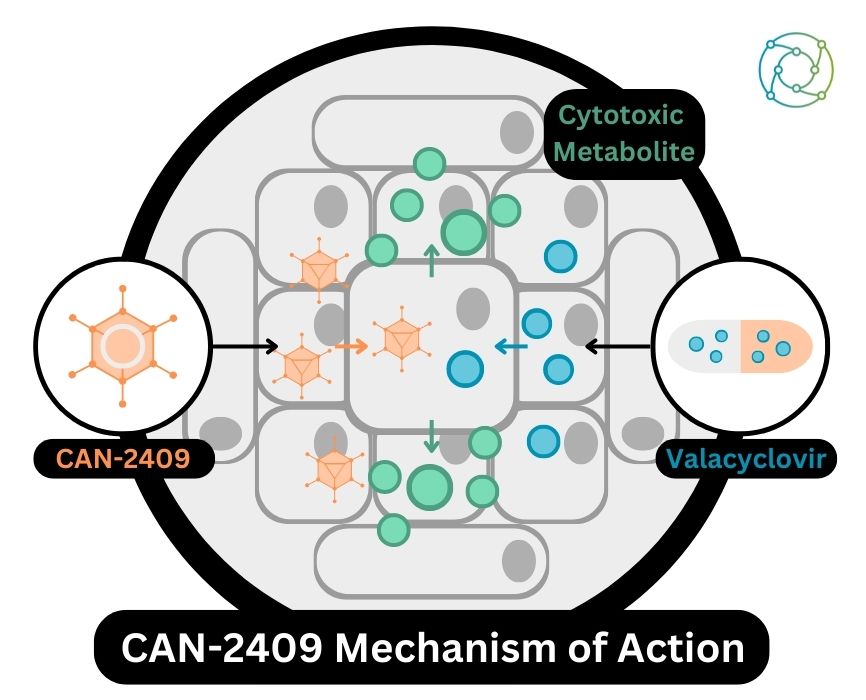
CAN-2409 Mechanism of Action
Talking the audience through the administration process and mechanism of action for CAN-2409, Tak explained that a gene-encoding thymidine kinase enzyme was administered at the site of the injection. “We treat the patient with the course of a small molecule – a tablet – called valacyclovir, which has been developed by GSK in the past,” he said. “If you administer valacyclovir in the presence of a thymidine kinase enzyme, it leads to the formation of a toxic metabolite at the site of the expression, which then leads to highly immunogenic cell death.” Subsequently, a variety of cancer-immune antigens specific to the patient’s tumour are released.
- Read: Oxford Globa's R&D Key 40 Stories 2022
- T Cell Enhancers: Conditional Activation for a Safe and Durable Response
- Circumventing Immune Escape via Bispecific T Cell Engagers and Synthetic Immunity
Tak added that a strong proinflammatory signal was present due to the use of an adenovirus to deliver the gene. “In other words, we’ve created optimal conditions to immunise the patient against their own variety of cancer-immune antigens.” The result is a local, CD8 positive T cell-dependent response against the injected tumour, followed by a systemic anti-tumour immune response. In the context of viral immunotherapy, CAN-2409 effectively teaches the body’s immune system how to fight cancer in injected tumours and uninjected metastases.
Case Studies in Viral Immunotherapy
Having established the fundamental principles of operation for CAN-2409, Tak moved on to discussing some case studies for its efficacy. One example was a mouse model of prostate cancer. Cancer cells were implanted in the mouse’s flank and injected intravenously to lead to the formation of lung metastases. “We only treated the tumour in the flank via the local application of CAN-2409,” said Tak. “As expected, we could demonstrate a very significant decrease of the tumour volume in the flank.” There was also a demonstrable decrease in the size and number of uninjected distant metastases.
“We only treated the tumour in the flank via the local application of CAN-2409... as expected, we could demonstrate a very significant decrease of the tumour volume."
Co-treatment of the mice with an anti-CD8+ depleting antibody further demonstrated that this anti-tumour response is mainly medited by CD8 positive cytotoxic tumour infiltration lymphocytes. “We have shown in a mouse model of lung cancer that if you take the circulating CD8 positive T cells after CAN-2409 treatment and you inject them into another mouse with the same tumour model but in the absence of CAN-2409 treatment, that these T cells have maintained their capability of recognising and killing the tumour cells.” In other words, this represents clear evidence of vaccination against the tumour.
The induction of CD8 T cell infiltration in the tumour has been replicated in a proof-of-mechanism clinical trial in patients with newly-diagnosed NSCLC. Discussing new clinical trial data, Tak focused on a cohort of 20 NSCLC patients with progressive disease in spite of continued immune checkpoint inhibitor treatment. “Most patients showed a decrease in the tumour,” he said. “We could show in this cohort of progressions that we were able to achieve disease control in 85.7% of patients – we expect new data shortly.”
CAN-2409 Findings and Takeaways
One major takeaway from Tak’s research was that CAN-2409 increases the local and systemic frequency of cytotoxic T cells. “We saw a very clear increase in infiltration by cytotoxic T-lymphocytes after CAN-2409 treatment associated with a reduction in the tumour mass,” he said. “We did a proximity analysis and we could show that these CD8+ T cells moved closer to the tumour cells where they will be able to do the job of killing tumour cells.” The treatment initiated the release of cytotoxic enzymes in the tissue which can induce apoptosis within the tumour cells. A steep increase was also visible in the number of CD8 positive and Ki67 positive granzyme T cells in the peripheral blood.
Rounding off, Tak added that CAN-2409 is generally well-tolerated in phase II clinical trials in patients with prostate cancer. Most patients were shown to tolerate intra-prostate injections the same as or better than prostate biopsies. The safety and tolerability of CAN-2409 administered with a prodrug has been demonstrated in clinical trials in over 950 patients with cancer, including cancers of the lung, pancreas, prostate, and brain. “It’s safe and well-tolerated,” concluded Tak. Clinical evidence supports the ongoing clinical trials of CAN-2409 across various solid tumours, and the prognosis for viral immunotherapy looks healthy. “This is a new modality, and we’ve started to see the first transformative clinical responses.”
Want to gain access to the full range of content across our Immuno portal? Check out our membership options to take part in our monthly discussion groups and other exclusive content. If you’d like to learn more about our upcoming Immuno UK: In-Person conference, visit our event website to download an agenda and register your interest.