Perfecting Precision Medicine: The Promise of Biomarkers and Spatial Biology

In recent years, the adoption of precision medicine has radically altered the immuno-oncology and pharmaceutical fields for the better. As a rapidly growing global market, it was valued at 52.4 billion USD in 2020 alone and is expected to increase at a CAGR of 11.5% between 2021 and 2027 to reach over 112.8 billion USD. Precision medicine provides a targeted and tailor-made approach to therapeutics, preventing or delaying treatment options with less favourability and tolerability profiles. Other advantages include its ability to facilitate patient-focused care and rehabilitation. Key industry players in the field include Merus, Labcorp, Hoffman La Roche, Thermo Fisher Scientific Inc, and Quest Diagnostics, to name a few.
Key drivers in such market growth include technological advances in characterising patients’ genomic and cellular profiles and the optimisation of cancer biology. However, precision medicine still requires perfecting. Understanding the spatial relationship between immune system cells and tumour cells to find prognostic biomarkers is essential to developing the next generation of precision practices. So too is understanding the progression of the tumour microenvironment to select the next best treatment option. In this article, we sat down with senior representatives from the University of Michigan, the Beth Israel Deaconess Medical Center / Harvard Medical School, and The Institute of Cancer Research (ICR) to discover more about the promise of biomarkers and spatial biology for the future of precision medicine.
Spatial Biology: The Context Defining Strategy to IO
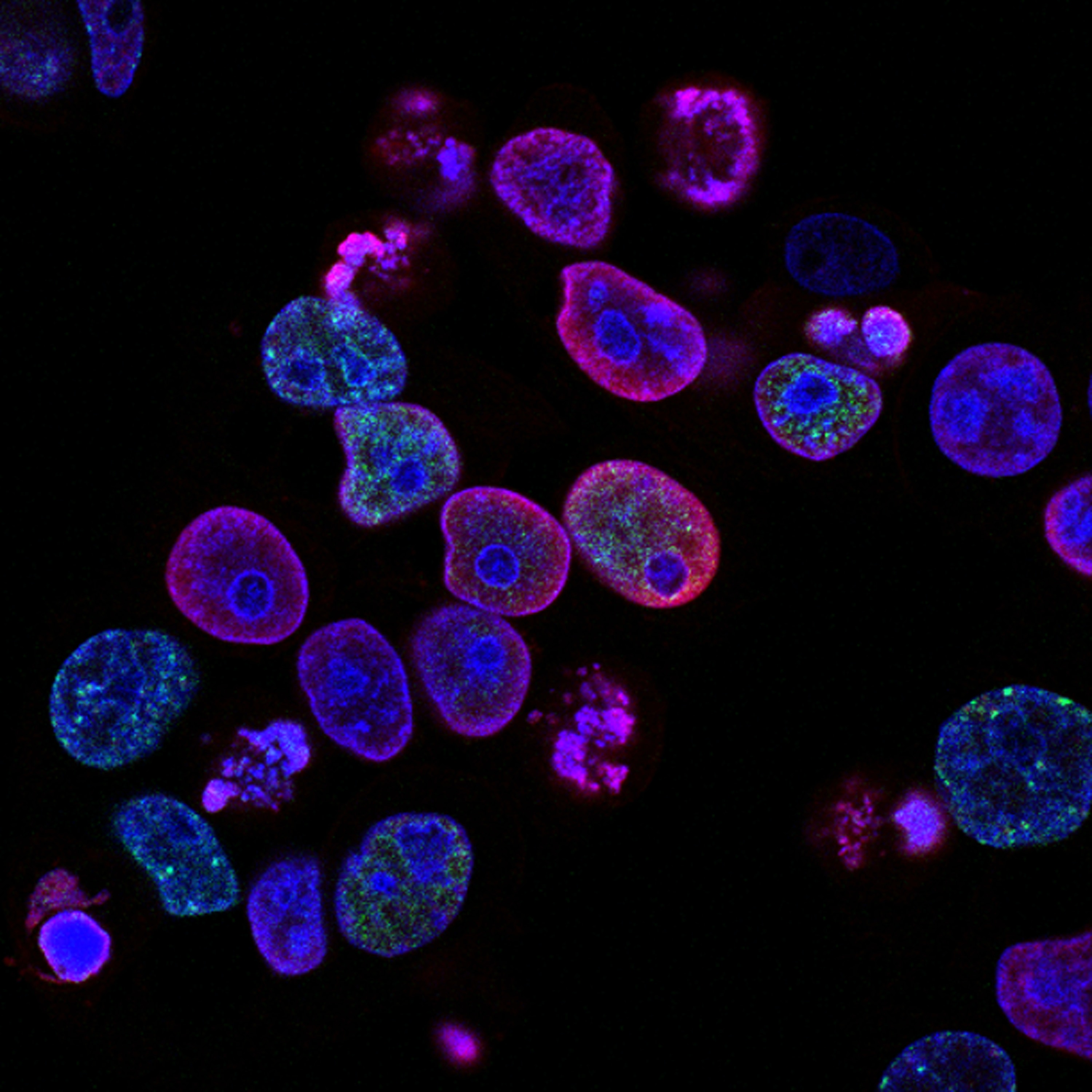
Spatial biology is critical for the understanding and measuring of disease and the requirements of precision medicine. Arvind Rao, Associate Professor of Computational Medicine and Bioinformatics & Associate Director of Bioinformatics for the Rogel Cancer Center at the University of Michigan, explained how “spatial transcriptomics is the modality in particular that harnesses the power to characterise the biology of cancer and derive important insights even from limited samples”, essentially leveraging spatial organization and context to ‘borrow information’ between single cells. In combining the study of histology and sequencing, spatial science provides an assessment of both the tumour microenvironment and cellular diversity of a sample. The functional role and contribution of the tumour microenvironment in cancer development supply key knowledge of the communication process occurring between cancer cells and their context. This in turn provides an integral insight into improving therapeutic responses.
As Ioannis Vlachos, Director of Spatial Technologies at the Beth Israel Deaconess Medical Center / Harvard Medical School, puts it, “what you will really want to know is about the cell states’ and co-localisation”. It is “not only their localisation but their core localisation and how they affect their immediate environment that we need to know,” he continued. As a mechanism of interaction between cancer cells and their ability to evade immune surveillance, microenvironments may well provide the key to advancing immuno-oncology treatments – and might even facilitate the targeting of solid tumours, which have proven tricky up until this point. Importantly, spatial biology also leads to identifying predictive tests in oncology. Prognostic biomarkers are used to accelerate and advance the efficacy of precision immuno-oncology therapies by identifying the presence or upregulation of a specific protein found on the tumour cells, which will correlate to a successful or unsuccessful immune response.
Perfecting Precision Medicine: Overcoming the Challenges of Spatial Biology
To iterate Vlachos, “the limit of science is essentially the limit of the available technology” within precision medicine. He explained the paramount need for optimised technology to develop predictive biomarkers and implement the next generation of precision medicine. Leveraging technology to investigate the interplay between cancer and the immune system requires the identification of disease and prognostic signatures in space. These techniques include genomic digital profiling that uses barcoding technology to retrieve digital readouts and high-throughput multimodal genome-scale imaging strategies.
While the study of pathology and molecular profiling have revolutionised the industry’s perspective on immuno-oncology and disease, progress and advancement remain a priority. Rao highlighted the need for an intricate approach to data analysis and interpretation. “With spatial data, you can specify and characterise information such as ligand-receptor cross-talk and cell-cell communication, leading to a more nuanced and context- sensitive network inference compared to bulk data analysis,” he explained. Rao continued by saying, “Implementing a detailed and mechanistic technology platform requires refinement of sophisticated models and corresponding networks to progress historical practices of data acquisition.” Anguraj Sadanandam, Founding Director of the Centre for Global Oncology, at the ICR agreed with the assessment, stating that “spatial and single-cell analysis provides much more clarity than what we have had previously.” The move away from reductionist bulk data analysis will provide exciting new opportunities for prognostic and predictive biomarker discovery in immuno-therapy.
Future Milestones:
According to Vlachos, “the most exciting thing about spatial biology right now is that we are just scratching the surface”. Despite being relatively new, the field is rapidly advancing and has already contributed to novel insights into cancer and many other diseases and disorders. “We have yet to see the full potential of what a comprehensive understanding of spatial context can do,” continued, Vlachos. The industry will need to reach several key milestones to fully welcome in an era of precision spatial medicine application. Prerequisites include automation for large data handling, reliable quality control (both for data acquisition and analytic pipelines), streamlining regulatory standards worldwide as well as accelerating timelines for clinical applications to reduce cost and significantly impact efficiency. An exciting future lies ahead and at Oxford Global we will look forward to seeing the results.
Want to find out more about the latest Immuno news? Register now for our May 2022 Preclinical Immuno-Oncology: Online to stay up to date with the latest models and approaches impacting preclinical workflow.