Human Stem Cell Model Development for Neurodegenerative Diseases
Human stem cell models for neurodegenerative disease provide a transformative system for target validation and pathway discovery. In particular, human-induced pluripotent stem cells (iPSC), which have been derived from skin or blood cells and reprogrammed back into the embryonic-like pluripotent state, provide a robust experimental endpoint for treating neurodegenerative diseases. In this Insight Article, Clare Jones, Chief Scientific Officer at Talisman Therapeutics, outlines the various strategies available for harnessing flexile human stem cell models to support drug discovery for neurodegenerative disease.
- Understanding Disease Progression with 3D Model Development
- Tackling Tumours In-Vitro with 3D Cell Cultures
- “3D model development is the Holy Grail of next-generation therapeutics”
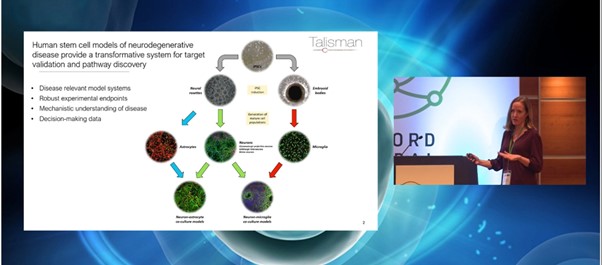
Among the most promising and translationally-relevant in vitro systems for neurodegenerative disease are iPSC-derived neuronal and glial models. These models offer a platform to enable the study of mechanisms underlying disease as well as being robust enough to support the generation of decision-making data. Jones explained how at Talisman, “we maintain our own bank of a variety of iPSCs which we then differentiate into different cell types, either down the neuronal lineage to make astrocytes and neurons, or via embryoid bodies to generate microglia.”
Application for Human Stem Cell Models
Understanding the different applications of stem cell models for neurodegenerative disease research is key to understanding Talisman's mission. As Jones pointed out, “the stem cell models form the centrepiece of all of the work we carry out at Talisman.” In addition to smaller scale mechanistic studies and compound profiling, Talisman also has a focus on phenotypic, small molecule, and genetic screens.
Human stem cell models for neurodegenerative disease provide a transformative system for target validation and pathway discovery.
These systems provide a flexible target and pathway validation set up to support drug discovery. In pairing the systems with an analysis of disease biology, Jones explained how they are robust enough to allow compound profiling and ranking as well as generating data that can feed into dose predictions. Whilst many of Talisman's projects to date have been based on small and large molecules, the model systems are not modality specific and are well-suited for testing novel gene and oligonucleotide therapies.
Why Human iPSC-Derived Neurons?
Neuronal dysfunction is a key feature of most neurological diseases. Therefore, having the ability to study human neurons in an in vitro system is critical to the advancement of drug discovery projects. However, primary human neurons are not readily available for study; patients do not donate neurons as they would blood samples.
Because of this, Talisman relies on a human iPSC-derived neuronal model to provide disease-relevant data to support drug discovery. This is a proprietary method based on published protocols and facilitates the generation of cortical neuron-astrocyte cultures. “We use protocols that model the in vitro differentiation and mimics in vivo human neurogenesis,” Jones added. Talisman studies the neurons at around 60 to 100 days in vitro and has found the cultures to be scalable.
Why Human iPSC-Derived Microglia?
Microglia are the immune cells of the central nervous system, and like iPSC-derived neurons, are not readily available for sample testing. Microglia dysfunction likewise features as an important factor in a wide range of neurological and neurodegenerative disorders. “Human iPSC-derived microglia are well characterised, phagocytically active, and respond to a range of stimuli,” Jones explained.
Human iPSC-derived microglial and microglia-neuron cocultures provide a promising system for the exploration of cell-to-cell interactions and neuroinflammation.
Both human iPSC-derived microglial and microglia-neuron cocultures provide a promising system for the exploration of cell-to-cell interactions and neuroinflammation. “Microglia-neuron cocultures are becoming increasingly understood and researched due to their well-defined changes and the communication between the microglia and the neurons.” In ongoing research, Talisman is developing triculture models that include astrocytes in addition to neurons and microglia.
Final Thoughts
Human iPSC-derived neuronal and glial systems are valuable and flexible cell models that support neurodegenerative disease research. In particular, human iPSC-derived neurons generated from disease-relevant genetic backgrounds enable cellular dysfunction detectable in patients to be studied in cell-based systems in vitro. Coculture models, provide exciting opportunities to study cellular functions and dysfunctions in a more complete and physiologically relevant systems. Perhaps what is most exciting within this area of advanced drug discovery for neurodegenerative disease is that available model systems are continually evolving to improve the translation value and utility of the data generated.
Want to find out more about the latest cell therapy news? Register now for Oxford Global‘s Cell UK: In-Person event to advance your understanding of cell-based products to ensure clinical and commercial success.