Immuno Oncology Investigations: The NKG2D Pathway, Diagnostic Biomarker, and Allogenic CAR Ts
The NKG2D pathway is a key component of the body’s immune system. Present in all NK cells and many other immune cells, NKG2D plays a role in triggering cytotoxicity, killing pathogens and cancerous cells. In this Insight article, we see how the NKG2D pathway may be an answer to the slippery problem of hepatocellular carcinoma diagnostic biomarkers. Furthermore, we learn how Celyad Oncology is engineering allogenic CAR Ts (including with CARs based on the NKG2D receptor) to be safe off-the-shelf therapeutics by impairing their TCR function.
Immune-related Biomarkers in Hepatocellular Carcinoma (HCC)
Nadia Guerra, Assistant Professor at Imperial College London addressed Cell UK 2021 on how NKG2D receptor-ligand interactions can act as prognostic markers for hepatocellular carcinoma (HCC). HCC is a common and deadly liver cancer, making up about 90% of liver malignancies, explained Guerra.
While most other types of cancer are decreasing in incidence, HCC is becoming more common over time. A background of persistent low-grade inflammation is responsible for this cancer indication, initiated by environmental factors like viral infections, alcohol abuse, or obesity.
Guerra explained that HCC progresses fairly fast and as a result has high morbidity. “We know of some risk biomarkers,” she said, “but these are late stage and low sensitivity.” Therefore, in order to improve patients’ chances of successful treatment, Guerra says that early diagnostic markers are needed.
Guerra reiterated the unmet needs from the field of study: to find biomarkers for the early stages of HCC; to better understand the tumour microenvironment; and to discover combination therapies for advanced HCC. Guerra then introduced the NKG2D pathway – an activating receptor triggered by ligands that are expressed on a wide range of tumours. Notably, the receptor is expressed on HCC at high levels.
“NKG2D is expressed on all NK cells,” said Guerra, and many others including: CD8+T cells, ??T cells, iNK T cells, and certain CD4+T cells. “The pathway can activate effector cells that are able to deliver cytotoxic hits against the tumour,” she said, adding that it also leads to the secretion of cytokines and chemokines in the tumour microenvironment.
There are multiple ligands for NKG2D also, eight of which have been identified in humans. Those ligands are rarely expressed in healthy cells, instead they are frequently upregulated in stressed, transformed, infected and autoimmune cells. Guerra explained that “this pathway is considered good as it promotes tumour rejection in most cases. But this is more complex in advanced HCC where it can also lead to the opposite effect at late stage of advancement of the disease”
In the case of HCC, all those ligands are expressed. Guerra reported that when grouping tumours based on their grade, grades 1, 2, and 3 which have elevated expression of most NKG2D ligands, happen to display p53 mutation and chromosomal instability. “As opposed to grades 4, 5, and 6, which are more associated with beta-catenine mutation in the CTNNB1 gene – those are less aggressive.”
“These studies suggest that those NKG2D ligands could be used as prognostic markers for HCC in combination with the genetic identification of the tumour, and they could help stratify patients that might better respond to immune checkpoint inhibitors.”
CAR T: Autologous and Allogenic
Laure Twyffels, R&D Manager, IND enabling group at Celyad Oncology also addressed the conference, explaining how her company have been investigating technologies enabling off-the-shelf (allogenic) CAR T therapy, and in particular how Celyad Oncology developed an allogenic NKG2D-based CAR T product.
Autologous CAR T Therapy
Despite the impressive accomplishments that traditional (autologous) CAR T therapy has produced, there remain areas for improvement, which allogeneic CAR T could improve upon. Autologous CAR T therapy derives T cells from the patient’s own blood before modifying them to express the desired CARs and infusing them back into the patient. The challenges with autologous manufacturing, Twyffels mentioned, are due to that the fact that it is produced and administered on a case-by-case basis. Furthermore, the delay between collection of the patient’s blood cells and the infusion of the CAR Ts (which is about 2 to 3 weeks) can be critical for patients with rapidly progressing cancers. “This limits the number of patients who can benefit from the therapy,” Twyffels said.
Another issue with autologous CAR T is that the T cells collected from the patient can be of varying quality. Different cancer patients will have leukocytes of variable fitness, depending on the patients’ immune characteristics and the therapies they have been exposed to. “A poor quality of materials collected from the patient may sometimes even result in failure for autologous manufacturing,” explained Twyffels.
Allogenic CAR T Therapy
In contrast, allogenic CAR T cells begin their life as T cells collected from a healthy donor, rather than being collected from the patient. Allogenic products are off the shelf and production can be scaled up – this decreases both cost-per-dose and the time the patient has to wait for treatment.
However, issues may occur by infusing foreign leukocytes into a patient. Allogenic CAR T redosing may be limited by immunisation of the patient against the allogenic CAR T, this is known as a host versus graft reaction – a rejection of the CAR Ts. Furthermore, Twyffels pointed out that there is also a “risk of the reverse phenomenon: graft versus host disease” (GvHD).
GvHD occurs when TCRs expressed on the transferred T cells recognise HLA molecules on healthy host tissues, Twyffels explained. Therefore, for allogenic CAR T cell therapy to be a stable and successful treatment, the TCR function on allogenic CAR T cells must be impaired.
Impairing the TCR Complex
In order to understand how the TCR function is impaired, it first needs be understood the structure of the TCR complex. As Twyffels explained, the complex consists of a variable TCR ? and ? heterodimer which is associated with a non-variable CD3 signal transduction complex consisting of ?, ?, ?, and ? subunits (see figure 1).
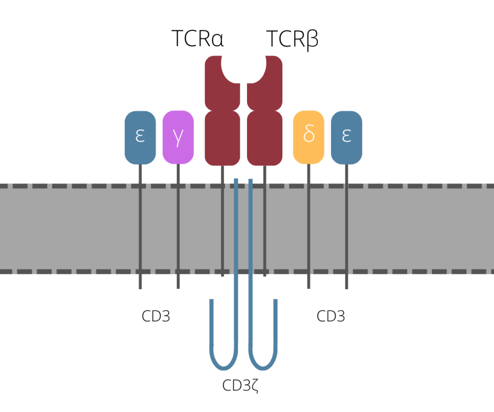
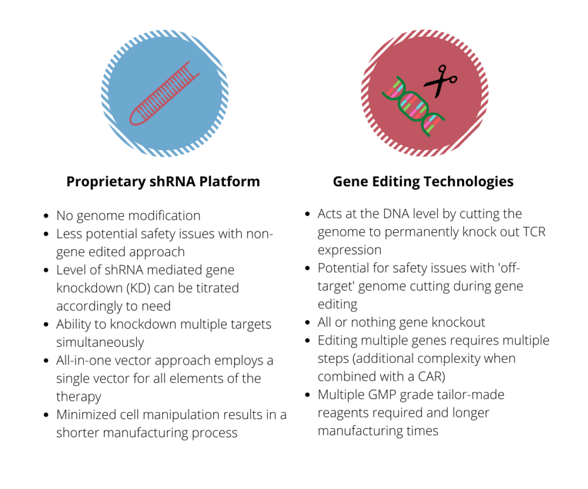
One way of halting the function of the TCR that is currently under clinical testing is to use gene editing - this approach is usually achieved by knocking out the gene that encodes TCR?. Twyffels explained that Celyad are taking a different approach, one that doesn’t use gene editing and targets CD3?. “CD3? is crucial for coupling antigen recognition to intracellular transduction pathways, and in addition is the rate limiting factor for the last steps of TCR assembly and export to the cell membrane” she explained.
CYAD-101
Their first approach – referred to as TCR inhibiting molecule 3 (TIM-3) - is based on the expression of a truncated version of CD3? with its ITAM signalling ability removed. “TIM-3 acts as a dominant negative form of CD3? which, when incorporated into the TCR–CD3 complex, deprives the complex of its signalling capability,” said Twyffels.
CYAD-101 is Celyad’s first product to integrate the TIM technology in an effort to block the function of TCRs on CAR T cells, and also the first allogeneic CAR-T product tested in the clinics for the treatment of solid tumors. The therapeutic is currently being evaluated in a Phase Ib trial for refractory relapse metastatic colorectal cancer. “CYAD101 has demonstrated a favourable safety profile at all evaluated dose levels, with no dose-limiting toxicity or GvHD observed in the fifteen patients treated.”
Another Approach: CYAD-211
Celyad have also worked on using RNA interference to block the expression of TCRs in CAR T cells. Their approach uses short hairpin RNA (shRNA) to “interfere with the expression of the TCR through targeting of the endogenous CD3? subunit.” Celyad targets this particular subunit because it can potentially offer them a greater level of control over TCR expression on the cell surface.
Greater understanding of the immune system and tumour microenvironments have proven to expand the capabilities of biomarker and drug development. Oxford Global is pleased to host innovators in this field in order to tackle the emerging and exacting challenges in immuno-oncology.
At Immuno UK: In Person: Gain valuable insights into the approaches impacting the immunotherapy field through 40+ outstanding presentations tackling key discussion points in immuno-oncology, immunology and inflammation.