Bispecific CAR-T Cell Therapies: Successful Applications in Mouse Tumour Models
Presented by: Colette Johnston, Vice President, Discovery at Crescendo Biologics Ltd
Edited by: Ben Norris
Targeting multiple tumour-associated antigens simultaneously could improve the outcome of chimeric antigen receptor (CAR)-T cell therapies by overcoming heterogeneous antigen expression and antigen loss. However, due to the instability of single-chain variable fragments (scFv) based CARs, it remains challenging to develop the simultaneous targeting of multiple antigens. Bispecific CAR-T cells, capable of simultaneously binding to two different antigens via heavy chain-only domains (“Humabody VH”) may provide the answer.
Colette Johnston, Vice President, Discovery at Crescendo Biologics Ltd, explained that her team’s research had focused on the generation of functional CAR molecules which regressed solid tumours at least as well as conventional scFv-based CARs. CAR-T cells are effective in confronting B cell and other malignancies, but heterogenous antigen expression and antigen loss remain persistent limitations of targeted immunotherapy.
As Johnston told the audience at Oxford Global’s Biologics UK 2022 conference, if a tumour no longer expresses the specific antigen that a treatment has designed to target, immunotherapeutic treatments can falter. Targeting two different tumour antigens via a bispecific receptor may be the key to new therapies.
The Crescendo Mouse and Humabody VH
The Crescendo Mouse is a transgenic animal which is incapable of making mouse antibodies and instead expresses human antibody fragments from an engineered transgene with diverse V, D, and J genes, the component parts of antibodies. The mouse produces fully human heavy chain only antibody fragments, known as VH. Johnston explained how the transgene effectively restores immune function in the Crescendo Mouse. She said it exhibited a normal level of circulating antibodies in the serum, as well as normal spleen architecture and B cell compartments.
- ADCs: The Next Generation of Biologics Therapeutics
- Discussion Group Report: Analytical Method Development for Biotherapeutics
- Industry Spotlight: AstraZeneca Buys T Cell Engager for $100 Million
Humabody VH are the smallest part of an antibody capable of specific antigen binding, about a tenth the size of a standard antibody. “The mouse responds well to immunisation,” said Johnston. “At this point, we’ve put hundreds of antigens into the animal, and we’re able to retrieve large numbers of highly diverse families of antibodies.”
VH are functional as single domains, and are ideal building blocks for a variety of antibody-based biologics, which have been a particular area of focus for Crescendo’s internal programmes. VH cells are well-suited to being the antigen-binding moiety of a CAR-T cell on account of their small size and lack of a light chain.
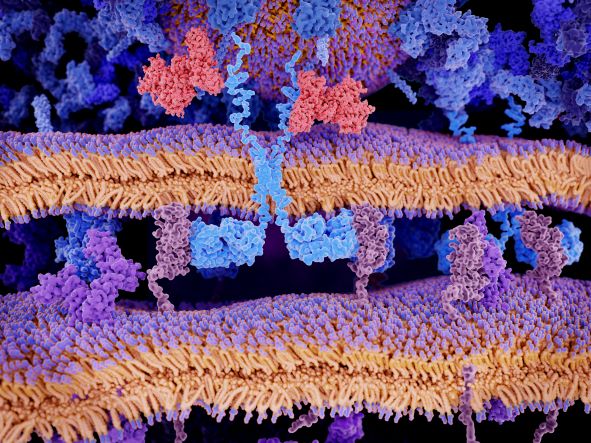
CAR-T Therapies: The Importance of Bispecifics in Combatting Antigen Escape
CAR-T cellular therapies have proven to be particularly effective against liquid tumours. “In particular, CD-19-directed CARs have had a huge amount of success and have really given some patients who had no other options a lot of extra life,” said Johnston. “Some of them go into remission with a really durable response that lasts for months and years – more than they ever would have expected.”
“CD-19-directed CARs have had a huge amount of success, and have really given some patients a lot of extra life.”
Johnston explained that one of the main reasons why patients eventually relapse after CAR treatment is down to the way in which their leukaemia expresses its surface proteins. “The tumour is often no longer expressing CD19 on the surface.” This is a phenomenon known as antigen escape, which means the CAR – which would otherwise be hugely effective – cannot recognise those tumour cells. If a CAR were designed as a bispecific capable of recognising two tumour antigens, this would guard against antigen escape and heterogenous tumour antigen expression by effectively offering ‘two shots on goal’.
Crescendo VH: Key Players in Effective CARs
In collaboration with Professor Gianpietro Dotti’s Laboratory at the University of North Carolina at Chapel Hill, Crescendo generated CAR-T cells using Humabody VH as the antigen binding part. CARs were directed to two different tumour antigens: prostate specific membrane antigen (PSMA) and mesothelin (MSLN), and control CARs were built using single chain variable fragments (scFv) recognising the same targets. “There were a number of criteria that had to be met,” she explained. “We needed to make sure we could get the CAR sequences into the cells and that there was a high level of expression on the cell surface.” It was also imperative to ensure that the T cells themselves were functional and capable of proliferation, and that the signalling pathways were intact.
CAR-T cells and tumour cells were co-cultured for five days in vitro. Johnston’s initial results using CARs recognising PSMA showed clearly that both the single chain and the VH killed cells which expressed the PSMA antigen, but did not kill cells that did not express PSMA. Cell killing was accompanied by T cell activation and proliferation. Subsequently, Crescendo carried out an in vivo assessment of PSMA CAR-T function in a mouse PSMA tumour model. Dosing with four million PSMA VH CAR-T cells resulted in complete tumour regression after 21 days.
A similar experiment in an MSLN tumour model showed that the anti-MSLN CAR with the Crescendo VH was far superior to the control scFv, even though the affinity for target is lower. “We are not sure why this is the case”, said Johnston. “It might be that higher binding affinity leads to over-stimulation of the T cells, driving them into exhaustion, and therefore a lower affinity antigen binder is more effective as a CAR-T.”
Humabody CAR-Ts: Conclusively Killing Tumours
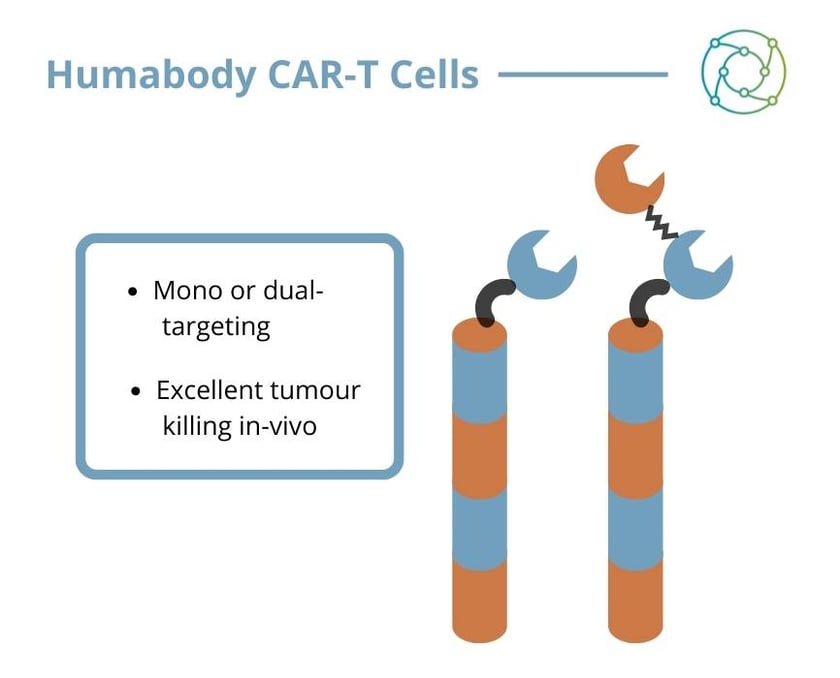
A final in vivo study compared VH CARs binding a single target to bispecific CARs in a ‘mixed tumour’ model where half the cells expressed PSMA and half the cells expressed MSLN. The PSMA VH was demonstrably successful in killing the PSMA cells, but eventually the MSLN cells grow out and the tumour progresses. The converse is true for the MSLN CAR. “With the bispecific CAR, you actually kill all the cells,” said Johnston.
Johnston rounded off her presentation by saying that Humabody VH are ideal for mono- and bispecific CARs. “We find that they’re extremely intrinsically stable, and that they’re very fit for purpose,” she explained. “Humabody-based CAR-Ts are comparable or superior to traditional single chain CARs both in vitro and in an in vivo mouse model.”
Bispecific CAR-Ts using Crescendo’s VH are capable of eliminating tumours with a mixed cell population. “As this piece of work goes forward, it could ideally be purposed in the clinic for the control of tumours with heterogenous antigen expression or where there is antigen loss”, Johnston concluded.
Visit our Biologics portal for more insights on CAR-T therapies. If you’d like to register your interest in our upcoming Biologics UK In-Person event, click here.