FDA Can Deem Animal Testing of Drugs No Longer Necessary

At the end of 2022, President Joe Biden committed to legislation that would see an end to mandatory animal testing of new drugs. The FDA Modernisation Act 2.0, which includes the Reducing Animal Testing Act, was signed by President Biden in December 2022.
The FDA has required preclinical animal studies of new drugs before they enter in-human trials since 1938. The legislation is part of a slew of new animal welfare laws signed by President Biden, including Acts that would end big cat petting, racehorse doping, and the exploitation of sharks for their fins.
Activists for animal welfare have welcomed the change. “These new policies will revolutionise the pharmaceutical world…” said Marty Irby, executive director at Animal Wellness Action.
Kentucky Senator Rand Paul who sponsored the new policy said, “the inclusion of this bipartisan effort is a step toward ending the needless suffering and death of animal test subjects.”
The Act does not ban the practice of animal testing out-right but does grant the option for researchers to use alternatives where available. Although the Act now permits the FDA to waive required animal testing, it is not clear yet whether they will make promotion to clinical trials without animal modelling a common occurrence.
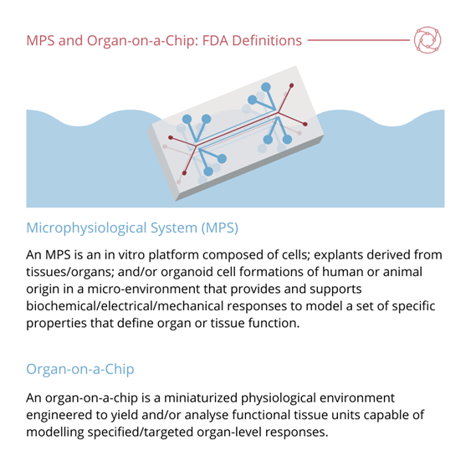
That being said, alternatives to animal testing have grown in efficacy and popularity over the last decade, with perhaps the most promising alternative being organ-on-a-chip modelling. Consulting group Roots Analysis has predicted last year that organ-on-a-chip market to grow at a CAGR of 21.3% until 2035.
Only one in ten therapeutics that make it to clinical trials will secure approval from the FDA, inaccuracy of preclinical prediction of in-human responses being a significant factor for this failure rate.
Harvard Professor and Founding Director of the Wyss Institute for Biologically Inspired Engineering, Don Ingber, said that “Animal models are wrong more often than they are right.” Ingber is also a board member and Scientific Founder of Emulate, a company that develops organ-on-a-chip technology. The pressure group Animal Wellness Action, who helped push for the law change, also managed to negotiate an additional 5 million USD for an FDA program focussed on reducing animal testing. The program, called the New Alternative Methods Program, will aim to develop and promote the use of alternate modelling methods.
Get your weekly dose of industry news?here?and keep up to date with the latest?‘Industry Spotlight’ posts.?For other Discovery content, please visit the?Discovery Content Portal.