Pharma Manufacturing: Digitalization and Industry 4.0

Digital tools to boost productivity and optimise pharma manufacturing are becoming increasingly prevalent through smart sensors, AI, ML, virtual reality, and cloud computing. Digital transformation is here for Pharma, and it is changing companies' priorities towards digital solutions.
Pharma Manufacturing: Falling Behind?
Research has shown that pharmaceutical companies have been reluctant to adopt digitalisation and new manufacturing technologies. As a result, the pharmaceutical industry is falling behind compared to other industries. While significant advancements have been made, defect rates, quality control and waste reduction remain relatively poor. This leads to increased manufacturing costs, which leads to reduced profits or higher prices for patients.
Despite this, there is a clear trend of manufacturing supply chains moving away from traditional paper-and-technology models towards digitalisation and automation. The drive for this comes from research, such as that from McKinsey, which shows that companies that embrace digitalisation can expect an average earnings increase of around 3.25%, a significant sum when considering the revenue of large pharmaceutical companies. Moreover, the benefits of digitalisation and industry 4.0 technologies have become increasingly apparent in recent years and, for most, hold potential too great to ignore.
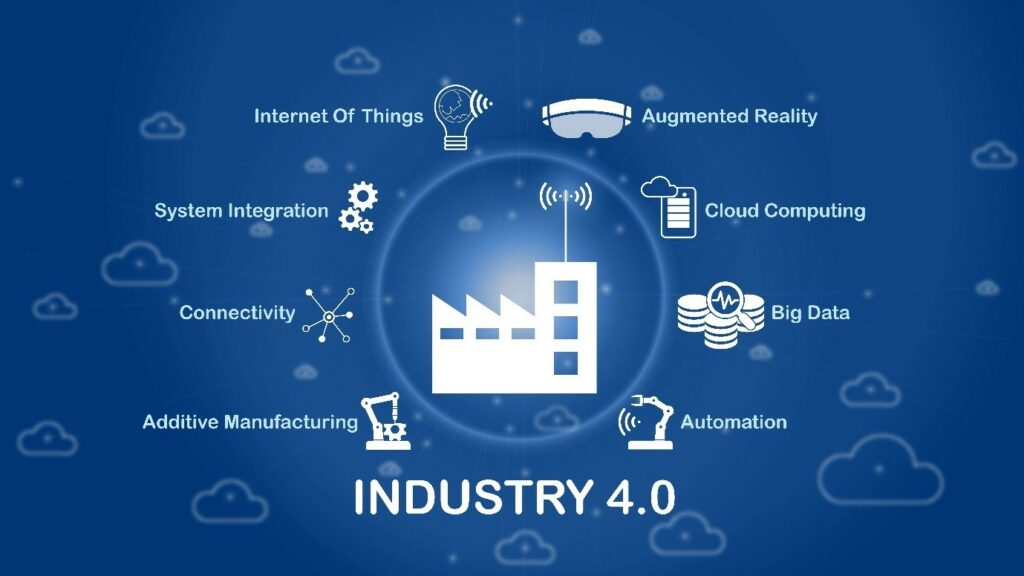
The Next Revolution: Industry 4.0 and Digitalization
Industry 4.0, also known as the fourth industrial revolution, is centred around digitalisation and connected technology. Artificial intelligence, machine learning, the Internet of Things (IoT), and blockchain are emerging technologies that are becoming increasingly important to stay competitive in the rapidly changing manufacturing industry. These all can potentially advance the way we manufacture pharmaceutics, particularly biopharmaceutics. The key feature of an Industry 4.0 workflow is the integration of connected devices, artificial intelligence, and robotics, which allows the automation of complex or repetitive processes as well as minimising or removing the need for human intervention.
Understanding The Challenges: Implementation, Data and Regulation
Most of the critical challenges of adopting Industry 4.0 technologies for pharma manufacturing are technical in nature and will require extensive investment and time to adjust and refine new practices, which is off-putting to many manufacturers. Companies will also need to improve their abilities to handle and optimise Big Data. Widespread adoption of Industry 4.0 will require cultural changes and innovations by manufacturers. Regulatory authorities will also need to adjust their practices.
Current regulatory frameworks will not be suitable for processing industry 4.0 environments. Slow regulatory updates can hinder advancement and encourage manufacturers to continue using conventional methods. The risk of a product not being authorised for sale due to regulatory framework incompatibilities with innovative new technology is too significant a risk for many companies, particularly in pharmaceuticals where the development process is both expensive and time-consuming. Existing Industry 3.0 technologies such as continuous manufacturing and adaptive control systems can still be an issue today.
While implementing many of the advanced technologies and manufacturing approaches for industry 4.0 will be difficult, the potential benefits are too great to ignore. Early research and testing have shown the possibilities of decreased costs, higher output, increased manufacturing safety, improved product quality,
Pharma Manufacturing: Looking to the Future
There is no doubt that pharmaceutical companies are taking the challenges of Industry 4.0 seriously. Digitalisation has become important enough that the FDA has begun to adapt their frameworks to fit the changing requirements. Last year, the FDA published a report that they are currently developing new rules for the use of artificial intelligence in medicine. While the digital transformation of the pharmaceutical industry is in the early stages, both industry and regulators are working hard to prepare for the next generation of manufacturing systems, data management, and computing technology. As the early adopters prove their value, these processes will become more refined and affordable, benefiting not just the companies but also patients who will benefit from safe, higher-quality drugs delivered sustainably and with a lower risk of shortages and defects. Oxford Global holds many events covering pharma manufacturing, smart labs, AI and more. For for information click here.






