Upstream Bioprocessing

Our May Discussion Group came together for an hour of specialist discussion about the current state and future directions of upstream bioprocessing for cell and gene therapy. This Discussion Group was a selected group of key industry leaders from various pharmaceutical companies and research institutions. Notable attendees included senior representatives from Novartis, UCB Pharma, PTC Therapeutics, and ViaCyte.
Amir Reza Goudarzi, former Engineering Head of UCB in Brussels (now Senior Director of Bioprocess Technologies at Bayer), led the discussion. He was joined by Matthew Coleman, Senior Engineer of Process Engineering Cell Culture at Genentech, Glyn Stacey, Gene and Cell Therapy Committee, International Stem Cell Banking Initiative and PIFI Fellowship, Institute of Zoology Chinese Academy of Sciences, and Williams Olughu, Principal Scientist and BPA Lead, Ipsen who were there to support.
Key discussion topics included analysing the resemblance between cell therapy and biologics, facilitating technology transfer for cell-based products, and establishing effective and efficient partnerships for upstream bioprocessing.
Industry Overview and Market Trends
Amir Goudarzi kicked off the session with a brief introduction to the current landscape of bioprocessing for cell and gene therapy. The overview focused specifically on the production bioreactor and touched on the typical challenges faced during biologics transfer technology (TT) whilst drawing parallels for the cell therapy field.
“When it comes to understanding the parallels in the technology transfers and in biomanufacturing, there are several cross-overs”
Amir Goudarzi outlined the potential steps in a cell therapy process (figure. 1). In his approach, he assessed the draw parallels between mAb processing and cell therapy to better understand potential synergies in industry capabilities.
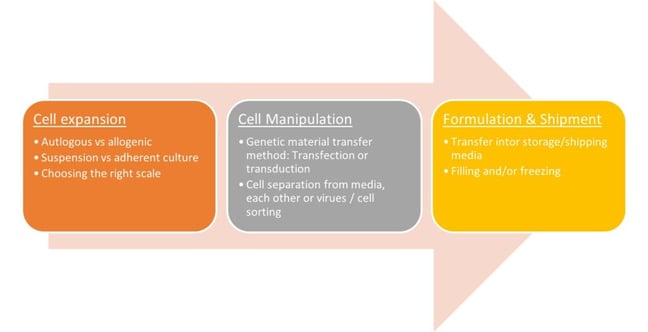
Figure. 1 – An Overview of Potential Manufacturing Steps for Cell Therapies
“When it comes to understanding the parallels in the technology transfers and in biomanufacturing, there are several cross-overs,” Amir Goudarzi continued. He identified that companies can keep building on their digitalization, automation, manufacturing platforms while embarking on this new cutting edge field. Cell therapy field often includes acquisition of highly innovative but smaller companies around the globe. Large tech transfer expertise in mAbs is also an asset in developing standardized manufacturing platforms to facilitate such developments.
“We are quite developed in this area in terms of learning from our mistakes and refining differing functional requirements and specification,” Amir Goudarzi explained. Single Use Systems (SUS) are also quite developed, however the practical regulatory understanding of them in CGT world is still ongoing. Most innovations here are in the processing steps definition, Analytics/PATs and End-to-end understanding of the impact of these choices on the overall process.
- Future Directions for Accelerated Upstream Bioprocessing
- Applications of Digital Twins in Refining Development Timelines
- Next Generation Cell Culture Process Development
Amir Goudarzi rounded off his introduction with the following statement: “there are many major learning opportunities and synergies happening in the cell therapy world compared to the mAbs world right now.” He projected that working with highly innovative global research teams, require developing some ‘modular concepts' to support reliable and reproducible scale up and/or transfers. Characterisation of manufacturing platforms (e.g. Bioreactors characterisation) is critical which enhances success rates in process development and increases industrialisation chances of molecules in the pipeline.
Genentech Case Study: Challenges and Successes in mAb Technology Transfer
Coleman continued with an in-depth case study on bioreactor TT and root cause assessments for critical quality attributes (CQA). The presentation started with an overview of the increasing complexity of products when conducting small molecule to cell therapy transfer (see figure. 2). Coleman reflected on the ways in which glycan-affected processes produce variability in cell culture performance and CQAs.
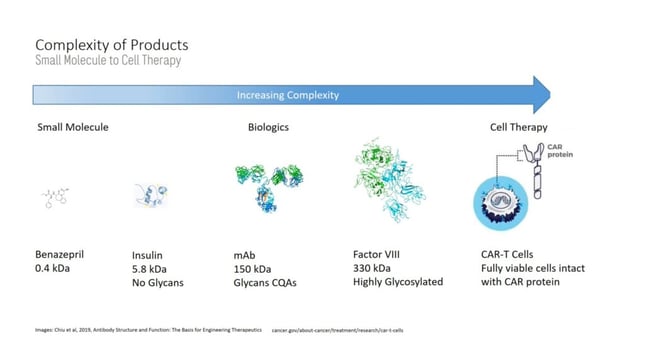
Coleman identified Genentech's 2020 transfer of commercial mAbs as a pandemic induced rapid transfer. “The onset of the COVID pandemic significantly shifted around the demands for specific molecules,” he explained. Whilst Genentech mAbs had a proven track record of commercial success, the events of 2020 required compressed timelines. The team found that an initial transfer in 17 weeks was made possible by the clearly defined and standardised approaches to basic cell culture requirements and quality control systems.
Relying on existing relationships with external partners such as the receiving site further facilitated what Coleman deemed “the fastest transfer I have ever worked on.” Rapid troubleshooting of CQA shifts involved in-depth study analysis of available Critical Process Parameters (CPPs) and utilisation of available cross-site data and experience. Ensuring optimal process data access requires precision culture monitoring at receiving sites and comprehensive summary statistics.
Identified areas of improvement include discontinuation of automated cell counting devices and review of detailed automation design at donor sites performed through surrogate process description. Coleman divulged how “interpretation of description was not coded correctly during the experiment, which resulted in subtle but impactful controls such as pH drift management and feed timing.” Additionally, the analysis could not fully evaluate donor site CQA variability to set comparability limits.
Q & A
Olughu then opened the floor for audience participation by inquiring about the best practices for CQA troubleshooting. “Why are problems not identified earlier? What steps can be taken to communicate potential drawbacks as early as possible?” he asked. One audience member identified including pseudo CQA steps whilst others debated the utility of such protocols for classifying critical attributes for process outputs.
Ensuring optimal process data access requires precision culture monitoring at receiving sites and comprehensive summary statistics.
Conversation then turned to the possibility of automation manipulation for expansion in cell and gene therapy. Stacey answered with the following statement: “the biggest benefit with automation is the reproducibility of manipulation.” In addition, he identified automation as a prospective solution to stability issues and cell responsiveness. However, potential drawbacks and considerations include the impact of novel culture vessel formats that may create different mass transfer effects in cell culture media and novel materials that could give rise to unexpected cell responses.
Concluding Thoughts
The discussion wrapped up with some final thoughts on the future of upstream bioprocessing for cell and gene therapy. With advancements being made to mitigate the risks and costs of appropriate technologies for the short and long term, rapid innovation is fast approaching the field. At Oxford Global, we couldn't have been more pleased with the turnout for our May cell Discussion Group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our Discussion Group series in June with a session focusing on ‘Gene Therapy Manufacturing'. Learn more about the Oxford Global Discussion Group series at our Cell Content Portal.
Want to find out more about the latest cell therapy news? Register now for Oxford Global‘s Cell UK: In-Person event to advance your understanding of cell-based products to ensure clinical and commercial success.






