Apical-Out Organoids: A Novel Strategy to Control the Polarity of Organoids Closing
Edited by: Cara Digby-Patel
Complex gastrointestinal in vitro models have multiple applications across the drug pipeline. They offer a better understanding of the diversity of patient biology and drug responses, and allow for disease modelling and compound screening. They can also answer questions related to ADME, help with biomarker discovery for diseases and pharmacodynamics, and provide a clearer understanding of drug safety.
At Genentech, work on various gastrointestinal in vitro models composed of primary tissue-derived organoids is being carried out. At Oxford Global's 3D Cell Culture: Online event in 2022, a senior representative from Genentech discussed the uses of gastrointestinal organoids, as well as Genentech's development of apical-out gastrointestinal organoids, a new type of model with a wide range of benefits.
Creating Gastrointestinal in vitro Models
Before discussing the various applications of gastrointestinal models, it is important to understand the process used to generate in vitro models. Researchers utilise cells harvested from tissue biopsies or resections to produce primary tissue-derived epithelial organoids (or spheroids) of human intestinal cells.
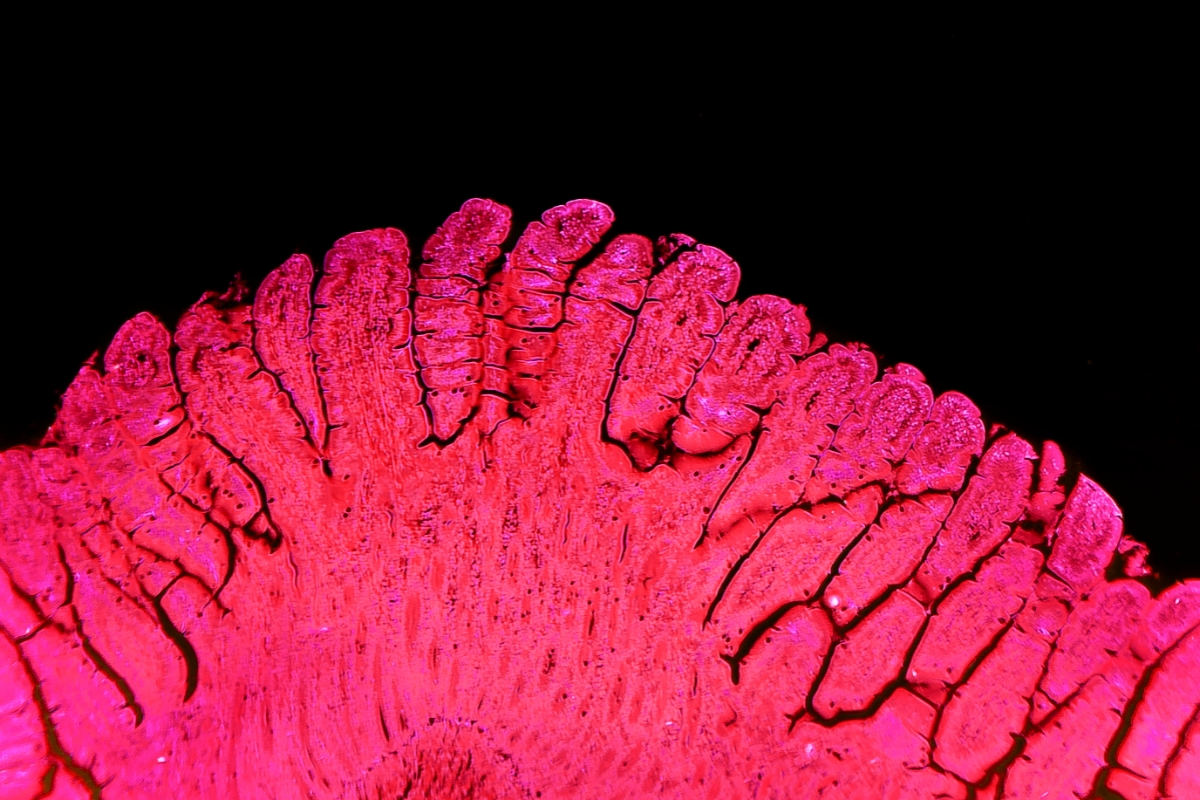
Crypts which contain stem cells are isolated and embedded inside a Matrigel or an extracellular matrix scaffold. When cultured with growth media, the intestinal stem cells grow into spheroids which can be propagated and cryopreserved. Much like the intestinal surface in vivo, the intestinal organoid is made of a monolayer of cells, as seen in the confocal image showing an organoid cross-section in Figure 1.
These models can be expanded, frozen, and propagated almost indefinitely. More importantly, they can differentiate into various epithelial cells, including enterocytes, goblet cells, Paneth cells, and enteroendocrine cells.
Genentech's Gastrointestinal Organoid Studies
Genentech is working on various in vitro gastrointestinal models using primary tissue-derived organoids in spheroid form, stem-cell state or differentiated state. In the talk, we heard an overview of three of these platforms.
The first utilised organoids for predictive gastrointestinal toxicity studies, which can help to assess drug safety during drug development. Researchers seeded gastrointestinal organoids in a 96-well plate and treated them with a dose curve of the compound of interest. There was an observable difference in the organoids, and researchers are now working on image-processing algorithms to quantify these images. Viability could also be measured as a readout of ATP levels using cell titer glow, and the data correlated well with animal studies.
- Video Q&A - In Conversation With…Stefan Przyborski, Professor, Durham University
- Understanding the Capabilities of 3D Bioprinting for Cell and Gene Therapy
- Human Stem Cell Model Development for Neurodegenerative Diseases
Another platform Genentech has implemented is the organoid-derived transwell monolayer. Organoid cells were disassociated from their matrix, broken into single cells, and seeded onto a matrix-coated transwell culture insert. The transwell monolayers require more cells per sample and a longer prep time than their organoid counterparts.
However, the transwell monolayer has a distinct advantage over other models: it provides access to both the apical and basolateral compartments, which enables polarity-specific dosing or analysis. It also allows an easier evaluation of barrier function, which can be measured by fluorescent tracer permeability studies or by measuring TEER, a measurement of electrical resistance across a monolayer.
In vitro models have also been used for engineered microphysiological systems. These can incorporate more complexity, such as flow or mechanical stretch. With more complexity, the models become more difficult to implement at higher scales, a challenge the field is currently addressing.
New Model Development: Apical-Out Gastrointestinal Organoids
A significant issue with standard gastrointestinal organoids is that it is challenging to access the apical surface as they develop with the basolateral surface facing outward. Apical epithelial access is vital for the study of several aspects of cell function and drug development. These include drug uptake and metabolism, host-microbiota interactions, innate immune responses, barrier function, and microbial pathogenesis.
Whilst transwell monolayers are more accessible, their disadvantages led researchers to look for an alternative way to access the apical epithelium by flipping the organoid inside out. This was achieved by removing Matrigel from the organoid growth culture, as this contains ECM proteins which initiate a signalling pathway which results in the self-forming and –maintaining basal polarity when integrins bind to ECM ligands.
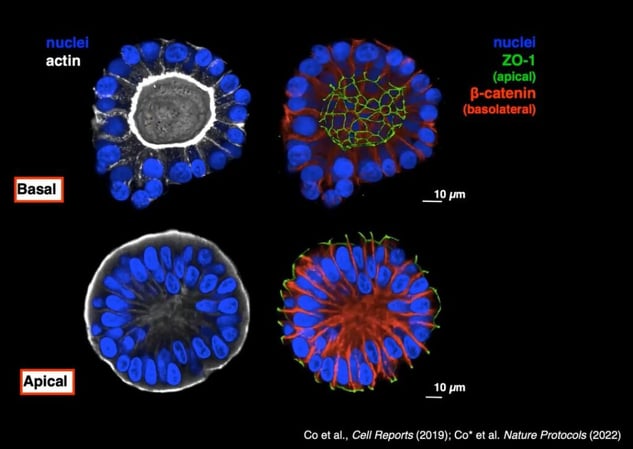
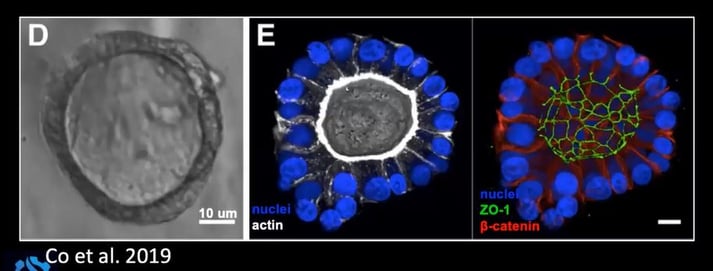
Researchers at Genentech confirmed the polarity inversion using the apical marker ZO-1 and the basolateral marker ?-catenin. As can be seen in Figure 2, ZO-1 moves to the exterior of the organoid when it is cultured without Matrigel.
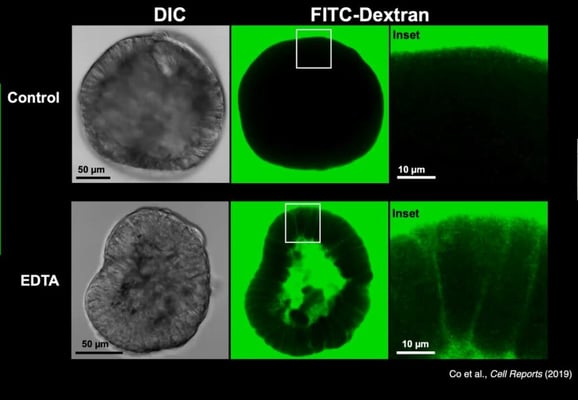
Apical-out organoids retained many of the critical functions of gastrointestinal organoids. When stem cell factors were removed, they could differentiate into various epithelial cell types. They also maintained barrier integrity, an essential function for all polarised epithelial cells; this was confirmed using fluorescent dextran, which is excluded from the organoid interior unless the barrier integrity was deliberately disrupted using EDTA (see Figure 3).
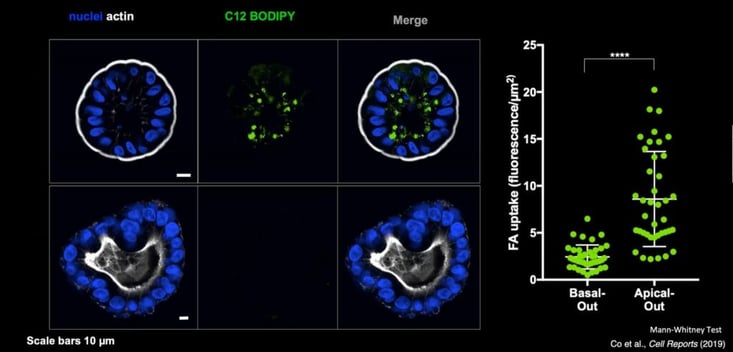
Apical-out organoids demonstrate in vivo phenotypic functions of gastrointestinal cells. For example, when looking at apical mucus secretion, researchers established that colon apical-out organoids secreted mucus (MUC2) out of the apical surface, whilst in gastric organoids they observed mucin secretion of MUC5AC. This variation between organoid types reflects that which is seen in vivo. Another aspect which reveals the utility of apical-out gastrointestinal organoids is their capacity to be used to investigate molecule uptake. Genentech designed an assay to examine the absorption of fatty acids in basal-out compared to apical-out organoids using the fluorescently labelled fatty acid C12 BODIPY. They established that only the apical-out organoids could apically absorb the fatty acid (see Figure 4). Researchers could easily adapt this assay to investigate the uptake of other molecules, such as drugs of interest.
These organoids are also especially useful when studying infectious diseases, as they enable live imaging across the apical/basal axis. This means that researchers can observe which bacteria are in contact with the cell. In one study, organoids were co-cultured with salmonella typhimurium, and researchers found that bacteria are better at entering through an apical surface. This result contrasts with previous literature, which suggested that there was no difference between apical and basal salmonella invasion. Old results came from studies conducted using transformed cell lines, which therefore highlights the importance of using primary-derived cell models.
The talk closed with a brief overview of some other ben
efits of apical-out organoids: the suspension cultures can be easily scaled up, a single well can be sampled at different time points, and organoids can be easily separated into different wells or condition-enabling screens. These models' benefits have been widely recognised, and they have already been adopted in several fields, including intestinal physiology and pharmaceutical evaluation. Moreover, Genentech is already looking into other ways to use this model within the drug development space.
Accelerating the adoption of 3D models in pre-clinical and translational research features as a key topic area of our next-in-series event, 3D Cell Culture 2023. Happening from 25th to 26 th April 2023 in London, UK, the programme agenda will be bringing together industry influencers to drive 3D model implementation and development for predictivity, validation, safety, and toxicity studies. This event is co-located with Organ Modelling UK: In-Person.