T Cell Engagement and Redirection – Strategies, Methods, and T Cell Design

Our February group came together for an hour of specialist discussion about the current state and future paths of T Cell engagement and redirection for biologic therapeutics. This discussion group was a select group of approximately 15-20 key industry leaders from various pharmaceutical and biotech companies, as well as renowned academic institutions. Gosia Nocula-Lugowska, Senior Principal Scientist at BioMedicine Design Pfizer, and W. Blaine Stine, Senior Director of Biologics Discovery Sciences at Abbvie, moderated the session. The discussion covered key topics from off-target binding for immune cell, T Cell, and NK Cell redirection-based therapies, to the importance of epitope binding kinetics and optimal geometry properties for engineering. Notable attendees included senior representatives from Boehringer-Ingelheim, Apogenix AG, GemPharmatech, Zumutor Biologics, and the University of Applied Sciences and Arts Northwestern Switzerland, to name a few.
Nocula-Lugowska and Stine kicked off the discussion with an overview of the T Cell landscape. Stine’s presentation was entitled ‘Off-Target Binding: You Don’t Know What You Don’t Know!,’ and explored the necessity of characterisation for T Cell engagement for ensuring that “antibodies bind not only the target that we want but also don’t bind to the targets that we don’t want.”
He summarised one of the foremost challenges of antibodies and antibody-based therapeutics as the issue of primary literature reproducibility. According to Stine, this is linked directly to the quality of the antibodies used in experiments. He explained how “oftentimes it is the inadequate level of characterisation that has led to a crisis when it comes to trying to reproduce a published study, which in effect fails when replicated in-house.” In particular, the presentation quoted an article by Egelhofer et al. which reported that in 2011 an evaluation of one-quarter of 246 antibodies used in epigenetic studies failed specificity tests because they were bound to more than one target (see Figure. 1). To overcome this, Stine spoke of the various solutions available, such as screening during antibody discovery to perform adequate characterisation that assesses the physical-chemical properties and target and off-target binding potential. One example included measuring off-target potential using high-density protein array technology to capture the complex nature of epitope-paratope interactions. The latter approach provides a streamlined and economical method for de-risking potential off-target binding.

Following Stine’s overview, Nocula-Lugowska presented on the optimisation of T Cell targeting of bispecific in what she titled ‘T Cell Redirection Strategies, Methods & Design.’ The presentation opened with a summary of the many advantages of T Cell retargeting and their unavoidable considerations. T Cell retargeting allows any T Cell to recognise the tumour antigen and are cytotoxic to dividing and non-dividing cells. They have the potential to proliferate at the site of activation and serially lyse target cells. Additionally, T Cell redirection strategies can target tumour cells with low
"The best way to retarget T Cells is to mimic, as much as possible, the naturally forming immune synapses between the T Cells and the tumour cells.”
- Gosia Nocula-Lugowska, Senior Principal Scientist at BioMedicine Design Pfizer
antigen expression. Nevertheless, multiple considerations need to be taken into account when designing T Cell bispecifics. Nocula-Lugowska started by explaining the excepted belief “that the best way to retarget T Cells is to mimic, as much as possible, the naturally forming immune synapses between the T Cells and the tumour cells.” Geometry is also integral to ensuring the viability of T Cell design and consequent toxicity, with 140 Å being highlighted as the optimal length. Other considerations include off-target binding, on-target and off-tumour toxicities, and the shedding of the tumour associated antigen and its structure. Nocula-Lugowska also pointed out that both the epitope and affinity can significantly impact the potency of CD3 bispecific.
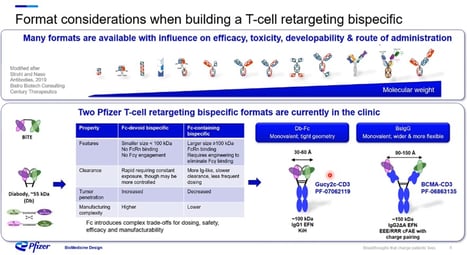
Following this, Nocula-Lugowska went on to claim that “when building a T Cell retargeting bispecific, many formats are available and can determine the developability and route of administration.” She identified the two T Cell retargeting bispecific formats currently being used for solid tumours in the clinic at Pfizer. The Db-Fc has tight geometry and usually requires engineering to improve folding and properties (see Figure. 2). The second format is a full-length IgG based on IgG2 isotype with higher flexibility between the paratopes. Both formats have Fc which produces a slower clearance rate and requires less frequent dosing, resulting in a more straightforward manufacturing process. However, the Db-Fc has decreased tumour penetration ability compared to the Fc-devoid bispecific BsIgG, which demonstrates increased tumour penetration despite its manufacturing complexity. Pfizer also found that the latter has a rapid clearance rate, requiring constant exposure, leading to a more controlled administration. Nocula-Lugowska summed up the findings with the following observation: “Fc introduces complex trade-offs for dosing, safety, efficacy, and manufacturability.”
“Tumours will often time downregulate tumour specific antigens, and so as an industry, we need to figure out how to get around this.”
- W. Blaine Stine, Senior Director of Biologics Discovery Sciences at Abbvie
After the introductory presentations concluded, the debate began. Stine opened the floor to discuss the different approaches to valency and how to leverage avidity to drive specificity. He continued by explaining how “tumours will often time downregulate tumour specific antigens, and so as an industry, we need to figure out how to get around this.” In particular, Stine highlighted the need to tackle low levels of expression to achieve combinations via avidity interactions to facilitate tumour specific targeting. Nocula-Lugowska responded by saying that “one way of achieving specificity for non-clean targets would be to have bivalent binding with low affinity.” This would mean that the bispecific will only bind to cells with a high expression level of the desired target. Methods to achieve this include bivalent binding to tumour associated antigens and monovalent binding to CD3. One audience member then spoke up to recommend the use of multivalent interactions, which involves one arm binding to one tumour associated antigen and the other arm binding to another tumour associated antigen. “However, the interplay between efficacy and toxicity varies depending on the receptors,” the audience member claimed. “There is no one rule or model that we can easily apply to this problem,” and concluded that it is, therefore, best practice to test for each and every molecule.
- Could Targetting Complex Membrane Proteins be an Understudied Opportunity in Antibody Engineering?
- Discover More About Optimising Receptor Trafficking with Bispecific Antibody-Drug Conjugates
- How can the Industry Advance Antibody Discovery, Optimisation, and Design?
The discussion continued with talk of dynamics during target regulation. In particular, audience members were interested to find out Stine and Nocula-Lugowska's experience with what they deemed the “target dynamics relating to efficacy”. Strategies of interest included engaging CD3 and CD28 in either one molecule or via simultaneous engagement. The panel responded by identifying the observations made during target dynamics as the main driver of such action. Nocula-Lugowska started, “you’ve touched upon two things; one is exhaustion of the immune cell that you’re targeting, which explains the inclusion of signal 3 cytokines, CD28, and 4-1BB, which can significantly help.” She continued, “secondly, because there are lots of tumour associated antigens, it is possible to eliminate those cells expressing your antigen, and then have a clonal kind of proliferation with lower levels, which will hopefully amount to a loss of tumour associated antigens.” Stine concurred, adding that “with these therapies, you need to think about durability of response.” He elaborated by saying, “one of the challenges encountered is the fact that you’ll get some initial very favourable data, but then due to the dynamics, you’ll fall short of obtaining the durable response you were hoping for.” Methods to overcome this include, implementing target assessment and engineering approaches or conducting NK cell engagement as a safer option.
The discussion group concluded with some final thoughts on the future of T Cell engagement and redirection. With ongoing research and development in T cell design, the future looks bright for this particular field of biologics. At Oxford Global, we couldn’t have been more pleased with the turnout for our February biologics discussion group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our discussion group series in March with a session focusing on Sustainable Synthesis of Therapeutic Oligonucleotides. Learn more about the Oxford Global discussion group series at our Biologics Portal.
Want to find out more about the latest market news and industry insights? Join our Antibody Engineering: Online event and delve into the foremost emerging technologies in antibody-based molecular engineering, novel & difficult targets, and antibody-based therapeutics.