Peptides Green Chemistry Strategies and Methods
Our January group came together for an hour of specialist discussion about the current state and future directions of green chemistry strategies and methods for peptides. This discussion group was a select group of approximately 15-20 key industry leaders from various pharmaceutical and biotech companies, as well as renowned academic institutions. Cécile Brocard, Head of Downstream Development, at Boehringer Ingelheim Regional Center Vienna, moderated the discussion. Boehringer Ingelheim is one of the world’s largest pharmaceutical companies with 40 globally licensed biopharmaceuticals to its name and a significant investment strategy in sustainable healthcare. Notable attendees and audience participants included senior representatives from Almac, AstraZeneca, Janssen, Novo Nordisk, Takeda, University College Dublin, St George’s University of London, and Northwestern University.
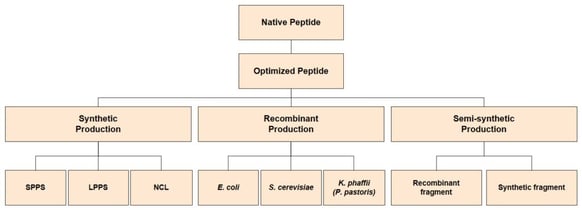
Brocard kicked off the discussion with a brief overview of the landscape with a presentation entitled, ‘Recombinant Peptides Expressed in E. coli: A Case Study’. During this, Brocard alluded to current market demands for peptides and delved into potential bottlenecks of peptide synthesis as well as recombinant opportunities. The presentation also touched on the recent industry evolution towards green synthesis and the modification and formulation techniques available for oral delivery. In particular, Brocard started by explaining how “there is no one size fits all approach or solution with biotherapeutic peptide synthesis”. This is because once the native peptide becomes optimised, there are three distinct approaches to synthesis available: synthetic production, recombinant production, and semi-synthetic production (see Figure. 1).
Recombinant expression is conducted within diverse cells in bacteria or yeast. Currently, there are approximately 150 peptides undergoing investigation in active clinical trials, with about 20% being produced recombinantly. Whilst chemical synthesis via synthetic production is often faster, most peptides are susceptible to proteolytic degradation and, according to Brocard, “are generally seen as difficult to express in cells”. She further explained how large peptides present a greater hurdle for chemical synthesis, as do their high environmental impact and the need for hazardous chemical solvents such as Dimethylformamide, N-methyl-2-pyrrolidone, and Trifluoroacetic acid. Recombinant production, on the other hand, provides a promising alternative to reduce manufacturing costs.
Brocard continued by delving into some of the various expression systems available for recombinant peptide synthesis. In particular, Brocard established how recombinant peptides can be expressed soluble either in E. coli or yeast via the secretion pathway. Another strategy for producing increased volumes of peptides includes using the form of inclusion bodies. Inclusion bodies are aggregates that form into bacteria and have the advantage of containing highly pure peptides and protecting peptides from proteolytic degradation. However, as Brocard explains, “this technique needs refolding and requires, for instance, chaotropic agents to refold the peptide in its native structure”. She continued by elaborating how these hosts are widely used for heterologous protein expression due to their genome being genetically well defined and easy to culture and modify. Other advantages include their fast expression with high-scale up capacity and their potential for high yields. “This means you can very quickly go from the laboratory scale to thousands of litres rather easily”, Brocard indicated.
Brocard concurred and acknowledged that tag-based purification of proteins is a widely used approach in basic research but has not yet been leveraged for industrial-scale practice due to technical and economic limitations.
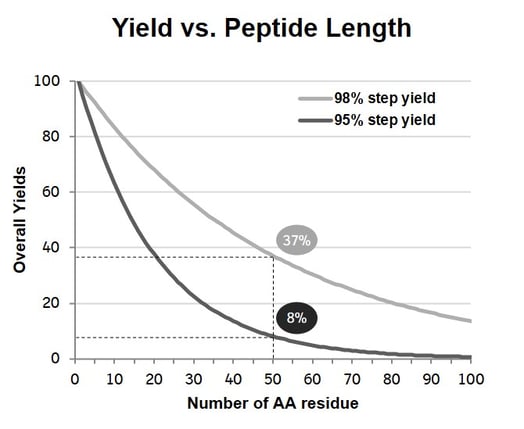
After Brocard’s introduction concluded, the debate began. One audience member opened the floor to discuss the fundamental challenges of biotherapeutic peptide synthesis. In particular, the said party inquired about how best to address and overcome the structural diversity of peptide synthesis. Brocard likewise stated that “there are certainly difficult sequences associated with hydrophobic peptides, in that they are difficult to express and difficult to synthesise chemically”. Contributing factors to the so-called difficulty can include the solvents and the purity and yield, which impact the sustainability and cost of the peptide synthesis. In response to the issue, Brocard drew the audience’s attention to a graph demonstrating yield’s rapid decrease with longer peptides with more than 50 amino acids residues when there is a 98% step yield in chemical synthesis (see Figure. 2). Conversation then turned to the various solutions to these challenges, with several audience participants recommending chemical ligation and tag-based purification. Brocard concurred and acknowledged that tag-based purification of proteins is a widely used approach in basic research but has not yet been leveraged for industrial-scale practice due to technical and economic limitations.
Following this, Brocard presented Boehringer Ingelheim’s CASPON Technology. CASPON is a proprietary manufacturing platform for proteins or peptides based on the expression of the protein of interest (POI) with a N-terminal affinity tag. The technology enhances expression levels through improved translation efficiency and systematic affinity purification. The platform aims to cleave CASPON tag with specificity and is applicable for expression in bacteria or yeast. Its construct comprises of an increased soluble expression tag, a tag for the capture step through an IMAC column, a linker to allow for flexibility, and a protease recognition sequence before the target sequence. According to Brocard, the procedure involves “the POI tag being adsorbed onto the column during the capture step, followed by the removal of the host cell protein to obtain a clean peptide”. Resultingly, Boehringer Ingelheim’s CASPON technology has been identified as fulfilling the criteria for industrial application.
Implementing a pre-emptive modelling approach ensures a degree of foresight, whereby chemists and clinicians can foster a comprehensive understanding of peptide fragmentation and troubleshoot potential pitfalls.
The discussion continued with how best to implement green chemistry into peptide synthesis. Debate centred on chemical waste production and the possibility of sustainable coupling, solvents, resins, and chemical ligation. One audience member breached the topic of mass intensity data to quantify improvements towards greener manufacturing processes. Brocard pointed out how cell culture conducted in bacteria reduces waste levels as “Expression in bacteria involves high-density culture, which covers a short time span”, she elaborated. On average, cell culture in bacterial expression takes three days. Other green technologies discussed included using modelling to overcome the challenges of solubility fragments. One audience member commented how “there are definitely tools out there to help solve the unpredictability of solubility”. And continued with, “one example is to computational model the size of proteins ahead of time”. Implementing a pre-emptive modelling approach ensures a degree of foresight, whereby chemists and clinicians can foster a comprehensive understanding of peptide fragmentation and troubleshoot potential pitfalls.
- Discover How to Advance Peptide Therapeutics for Personalised Cancer Vaccines
- Read our 2021 Market Report About the Latest Trends and Industry Challenges for Peptide Therapeutics
- Can AI-driven Peptide Purification Advance the Therapeutic Capabilities of Peptide Drugs?
The discussion group concluded with some final thoughts on the future of green chemistry for peptide therapeutics. With ongoing research and development and numerous exciting pipelines emerging in the field, the future looks bright for the peptide field of biologics. At Oxford Global, we couldn’t have been more pleased with the turnout for our January biologics discussion group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our discussion group series in April with a session focusing on Accelerating Antibody Engineering with AI/ML & Advanced Computational Tools and Technologies. Learn more about the Oxford Global discussion group series at our Biologics Portal.







