Peptide Discovery: Unlocking the Future of Disruptive Therapeutics
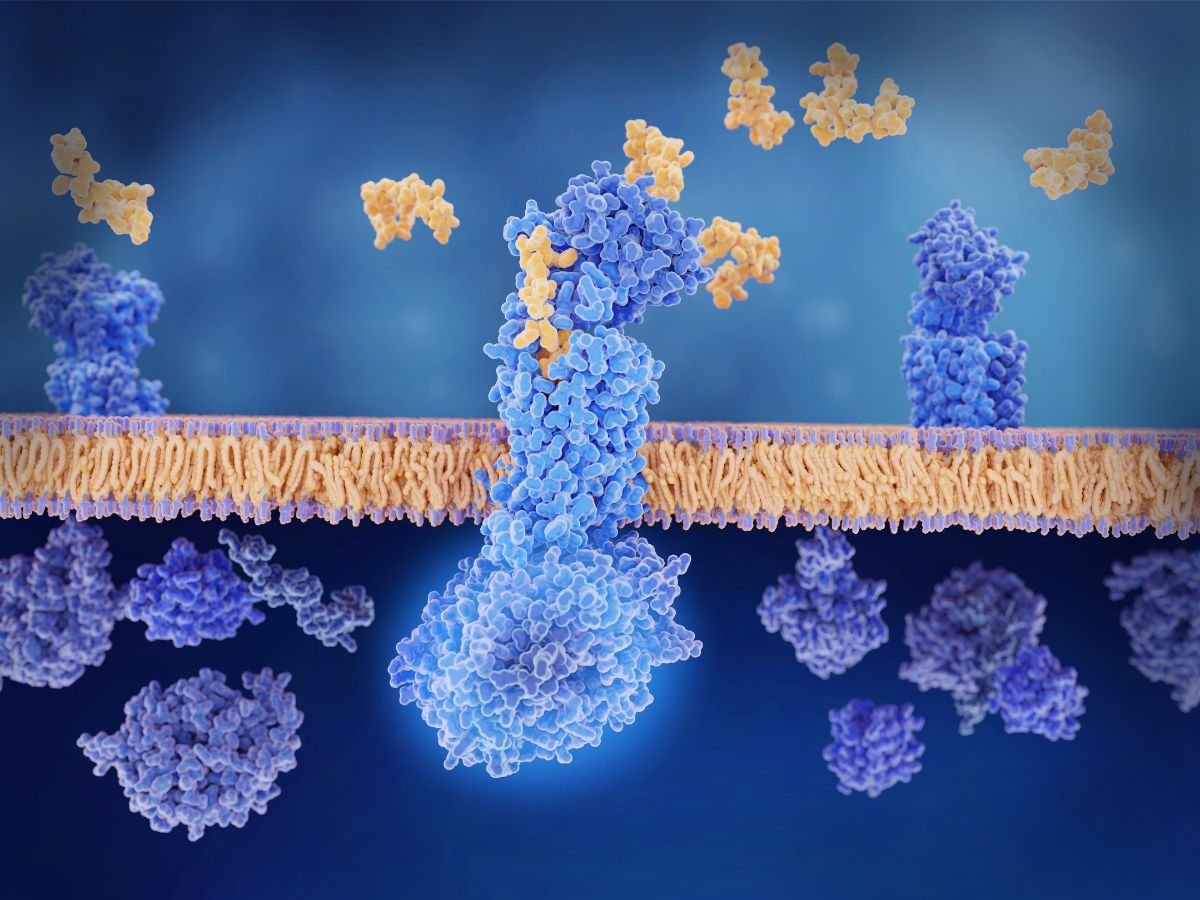
In 2021 the global peptide therapeutics market was valued at 35.9 billion USD. By 2027 it is forecast to rise to over 61.9 billion USD, at a CAGR of 9.4%. The astounding worth of the peptide pharmaceutical industry can largely be attributed to it being a relatively safe, well-tolerated, and less toxic form of treatment option.
Peptide therapeutics have consequently gained notable traction worldwide for combating illnesses such as cancer, infectious diseases, metabolic disorders, and neurological disorders. Pioneering areas of development in the field include the possibility of peptide precision medicine and a personalised cancer drug.
Innovative Peptide Discovery: A 3 Case Study Analysis
Since their discovery in the late 20th century, more than 7,000 naturally occurring peptides have been identified, and 80 plus peptide drug products approved for market. In 2021 alone, there were over 150 therapeutic peptides under clinical investigation.
But what is happening in the field of peptide drugs today? And what future-orientated steps are being taken by the industry concerning peptide discovery? We hear from 3 of the key industry players embarking on the next generation of pre-clinical peptide discovery.
1.) UCB - Harnessing Bovine Antibodies with Ultralong CDRH3:
UCB use knob domains to harness the diversity of the bovine immune system to generate peptides in vivo. Knob domains are the smallest antibody fragments identified to date, and bovine antibodies which possess ultralong CDRH3 present a unique opportunity to produce affinity maturated, cysteine rich peptides. For example, following immunisation, it is possible to isolate knob-domains from the antibody scaffold to create cysteine-rich autonomous peptides of approximately 4 to 5 kDa, with affinities in the pM-nM range.
“Knob domains are the first antibody fragments to be truly amenable to chemical synthesis,” Macpherson confirmed.
According to Alex Macpherson, Senior Principal Scientist at UCB, “this discovery method helps us address a broad range of potential challenges and different target proteins and architectures.” In particular, the immune system’s versatility has made epitopes that are planar and polar (antibody-like) or buried and hydrophobic (peptide/NCE-like) more accessible. Harnessing bovine antibodies for peptide discovery has also proven useful in addressing smaller target sites that are challenging to conventional formats.
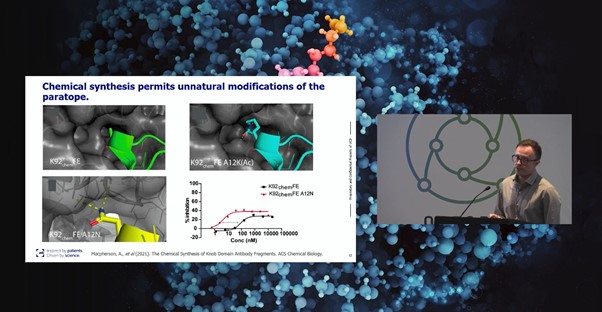
Figure. 1 - Alex Macpherson, Senior Principal Scientist at UCB, presenting at Biologics UK 2022
“Knob domains are the first antibody fragments to be truly amenable to chemical synthesis,” Macpherson confirmed (see Figure. 1). UCB’s discovery and engineering platform uniquely combines the diversity of the bovine immune system with the almost infinite diversity of chemical space. Immune methods for knob domain discovery include immunising cattle and enriching for antigen specific B-cells by flow cytometry. Alternatively, unusually for cysteine-rich peptides, phage display can also be used to derive binders.
The proximity of the N- and C- termini allows fully cyclic chemical fragments to be produced and enables a recombinant method for engineering valency into proteins, by targeting flexible loops as insertion sites. “We have fused knob domains into various loops of serum albumin, to extend half-life via binding to FcRn and have engineered non-binding loops of camelid VHH to produce a small, single chain bispecific antibody,” Macpherson continued.
2.) Bicycle Therapeutics - Discovering Penicillin Binding Protein Inhibitors Using a Modified Phage Display Platform:
Bicycle Therapeutics successfully discovered penicillin-binding protein (PBP) inhibitors using a modified phage display platform, offering unique opportunities for novel antimicrobials. The project aimed to develop a clinic-ready candidate for the treatment of E. coli, Klebsiella pneumoniae, and other pathogens of the enterobacterales. Using a platform based on bicyclic peptides, Bicycle Therapeutics found that the E. coli PBP3 target was highly tractable, with low nanomolar binders identified in phage selections. When the E. coli membrane was made artificially permeable by expressing pores to create hyperporinated strains, the Bicycle was able to inhibit bacterial growth in minimal inhibitory concentration (MIC) assays.
“It became clear that through linking to a peptide vector, our conjugates could achieve outer membrane uptake” Catherine Rowland, Team Leader at Bicycle Therapeutics, explained. Antimicrobial peptides (AMPs) offered a starting point for vector sequences selected from a Data Repository of Antimicrobial Peptides (DRAMP) database.
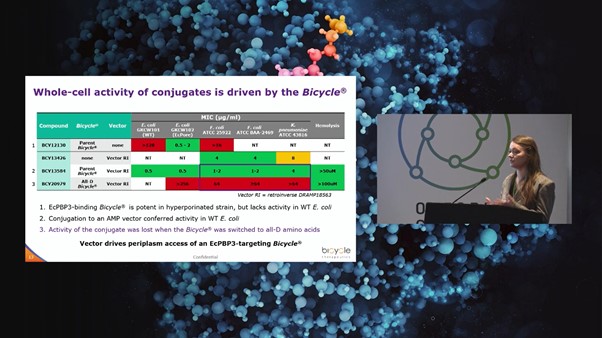
Figure. 2 - Catherine Rowland, Team Leader at Bicycle Therapeutics, presenting at Biologics UK 2022
Bicycle-AMP conjugates showed promising activity against wild-type bacteria. Rowland commented: “We saw that conjugate activity in bacterial cell killing was lost when the Bicycle was switched to all-D amino acids” (see Figure. 2). This suggested that in the conjugate molecule, the vector drives periplasm access where the E. coli inhibition by the Bicycle is then causing the cell death. The other encouraging data was the absence of hemolysis atrelevant concentrations.
- Discover How to Advance Peptide Therapeutics for Personalised Cancer Vaccines
- Read our 2021 Market Report About the Latest Trends and Industry Challenges for Peptide Therapeutics
- Can AI-driven Peptide Purification Advance the Therapeutic Capabilities of Peptide Drugs?
Alanine scanning revealed tractable positions for further stabilisation and potency improvement. Results from screening in a hyperporinated E. coli strain identified a tryptophan in loop 2 as essential for binding, whilst other residues such as a serine in loop 1 were tractable for substitution.
Structure-activity relationship (SAR) data and a co-crystal structure were used to tune the properties of the conjugates. Currently, Bicycle-AMP conjugates are being progressed towards a clinical candidate under a project funded by a Biomedical Catalyst award (Innovate UK).
3.) Sanofi - Peptide Mimetics of Human Relaxin-2 for Cardiovascular Disease:
H2-Relaxin is a double chain peptide with 53 amino acids which is connected by three disulfide bonds. It possesses an insulin-like structure and belongs to the relaxin family of peptides (see Figure. 3). Relaxin was initially identified as a pregnancy hormone as it plays a significant role in hemodynamic adaption and connective tissue remodelling during pregnancy.
Relaxin has shown positive results in heart failure phase 2 clinical trials but failed to meet its primary endpoints in phase 3. Sanofi set out to discover a lasting analogue for cardiovascular disease patients using by optimising Relaxin-2 effectiveness. “We believe this is due to the fact that Relaxin has a low and short duration of action which prevents it from being used as a chronic treatment,” Sergio Mallart, Principal Scientist at Sanofi explained.
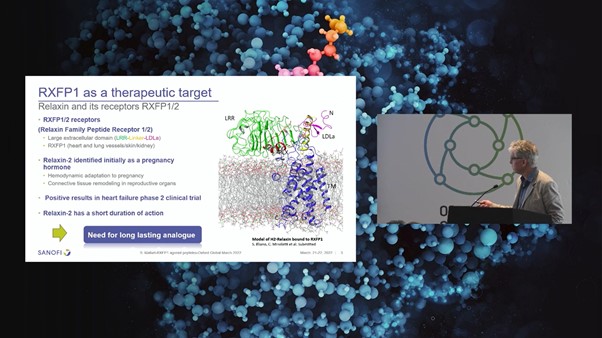
Figure. 3 - Sergio Mallart, Principal Scientist at Sanofi, presenting at Biologics UK 2022
Relaxin has two main binding sites: a high-affinity binding site on the LRR domain and a secondary binding site on the linker between the LRR and the LDLa module. Relaxin binds to RXFP1 mainly through the B-chain whilst there are no specific binding interactions between the A-chain and RXFP1 transmembrane domain. Starting with relaxin B-chain, Sanofi conducted multiparametric peptide optimisation and in vivo assays to extend the duration of action using peptide lipidation.
Sanofi set out to discover a lasting analogue for cardiovascular disease patients using by optimising Relaxin-2 effectiveness.
Overall, Sanofi showed that potent lipidated peptides could be obtained with a simplified structure compared to double chain analogues and which possess promising sub nanomolar activity in cellular assays. “These molecules are highly soluble, and there was no sign of aggregation, attesting to their chemical and physical stability,” Mallart commented. In vivo relaxin-like activity was also recorded, and Sanofi found that large animal pharmacokinetics predicts the potential for QD dosing in humans. “This new class of RXFP1 agonists can open the door to new treatments for chronic fibrotic and cardiovascular diseases”, he continued.
Future Outlooks:
The above-mentioned case studies provide an exemplary look at just some amongst an entire array of exciting discovery techniques unlocking the future of peptide discovery. As the field ushers in the next level of disruptive innovation, we can expect the success of the global peptide market to skyrocket. With the competitive landscape brimming with activity, at Oxford Global, we predict that the position of peptide therapeutics on the world stage will continue to shine.
Intrigued about the future of the biologics vaccine market? Register today for Vaccines Europe for an intensive 2-day in-person meeting, delving into the latest in vaccine research, platform technologies, analytics, and vaccine development.







