Surrogate Biomarkers in NASH and Liver Cirrhosis: Qualifying for Clinical Trial Validation and Legislative Success
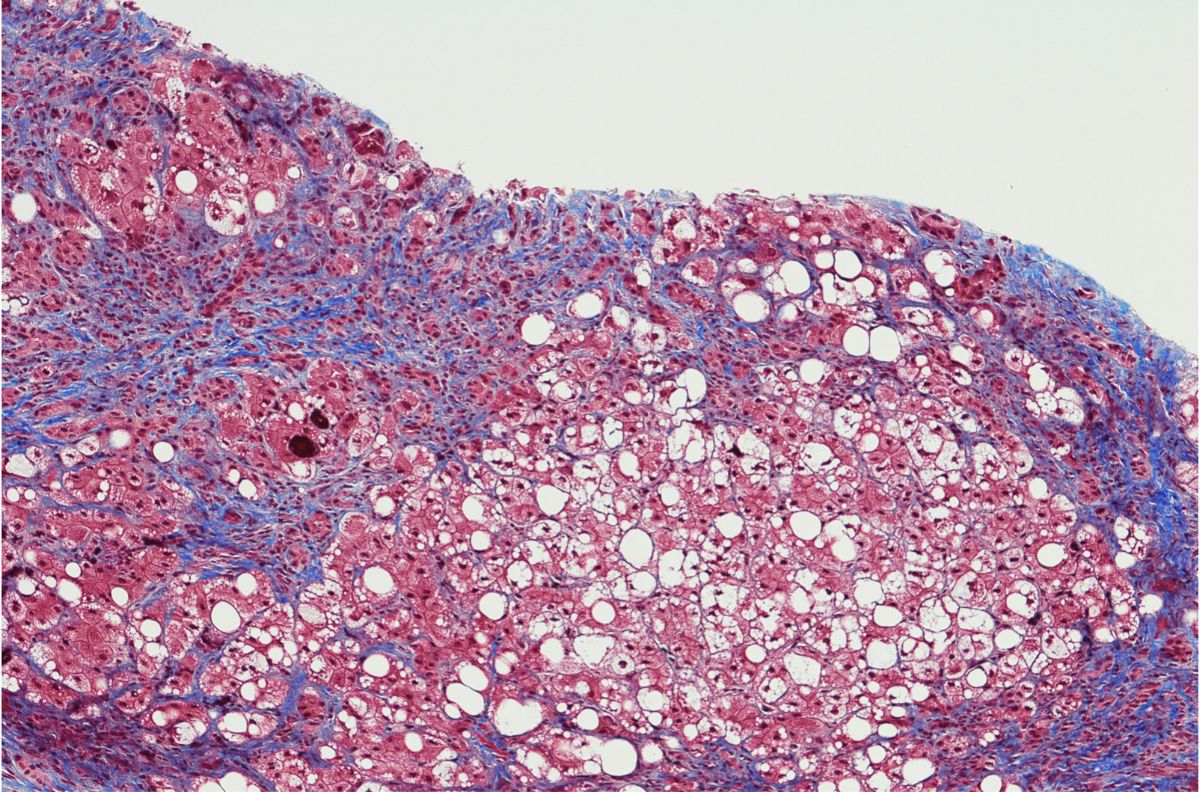
Surrogate biomarkers — substitutes for clinical endpoints in specific treatments — are often cheaper and easier to measure than ‘true’ disease endpoints. As one example, measuring a patient’s blood pressure is a much more straightforward indication of ventricular function than making them undergo screening to image their heart. The same is true of biomarkers for non-alcoholic steatohepatitis — known to the medical world as NASH. As an increasingly common cause of cirrhosis, hepatocellular carcinoma, and liver-related death (LRD), there is a critical need for effective drug therapy that improves clinically relevant endpoints.
The current landscape for conditional drug approval requires long-term follow-up to demonstrate a reduction in the more damaging endpoints of liver cirrhosis. Consequently, there is an essential need to develop non-invasive markers which are dynamic to drug-induced changes. One approach to further this has been the development of biomarkers as surrogate endpoints: indicators of treatment efficacy which are less invasive than direct biomarkers from liver biopsies.
To qualify as a surrogate endpoint, a biomarker needs to demonstrate a significant ability to predict a clinically relevant outcome as well as the effect of treatment on this outcome. It also requires validation from regulators such as the FDA and the EMA. Designing a clinical trial with the aim to satisfy every demand from a regulator right from the start can be an incredibly complex task. So what steps can be taken to help the process along?
Surrogate Biomarkers and Investigational Device Exemptions
When considering the first steps of a biomarker-driven approach, there are two key points to bear in mind: will the biomarker be usable in the clinical trial submission? And will it be used as an efficacy tool, confirmatory, or exploratory tool? Surrogate biomarkers fall into the latter category as a prognostic means of assessing liver-related disease progression, measuring one or more analytes obtained from human samples.
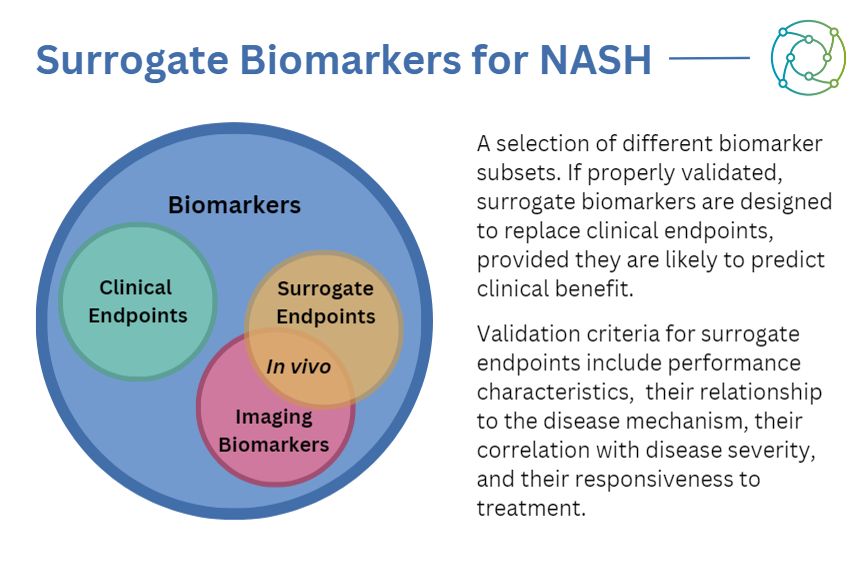
Some elements of biomarkers are used for exploratory purposes or analysis to circumvent seeking an extension for approval from the appropriate regulators. This is particularly relevant to investigational device exemptions (IDEs), which the FDA approves to permit the use of a device in a clinical study to collect data on safety and effectiveness.
Investigational use also includes the clinical evaluation of certain modifications or new intended uses of legally marketed devices. In the context of surrogate biomarkers for NASH, IDEs are used for exploratory purposes, retrospective analysis, or non-invasive sampling: they cannot be used to inform a treatment decision.
Considerations in the Biomarker Qualification Process
As an exploratory biomarker makes its way through the qualification process, it may be accepted for general use over an extended time period subject to the accumulation of scientific knowledge and experience. The qualification process is broadly the same in both Europe and the US, with the FDA and EMA following a similar approval pipeline. Both require a letter of intent (LOI), a qualification plan, and a subsequent full qualification package.
Currently, diagnostic screening approaches such as wet-markers are being used as a supplement to the screening process. Researchers in the field are using them to increase the chance of a successful histology. Any change to this approach which places histology as the front and centre diagnostic tool in a study will need a full qualification requirement described and approved by the authorities.
This may happen in the near future, bit a lot of discussion needs to be able to happen around wet-markers in diagnostic screening first in order to avoid the need for a biopsy. In this context, the best-practice approach would involve assessing the situation without subjecting the patient to a potentially painful and invasive procedure if it can be avoided.
Surrogate Biomarkers in NASH: Precision Medicine Approaches
Given the array of issues and complications posed by NASH, highly involved treatment scenarios and prognostics are required to properly assess best-practice approaches. A further complication from the regulatory standpoint is that some precision medicine approaches for NASH do not currently have specific guidance strategies. Predictive approaches need to be confirmed and validated with the right data, so a different effect is visible with homozygous and heterozygous samples.
One present area of interest in the field is pharmacodynamic (PD) biomarkers: molecular indicators of drug effect on a specific target in an organism. A PD biomarker can be a sign of treatment efficacy, which can signal the efficacy of a targeted treatment. While there is no specific requirement for the qualification of PD biomarkers at present, a shift in legislation may alter this further down the line.
Liver Histology and NASH Histology Endpoints
The end goal with the development of surrogate biomarkers in NASH and liver cirrhosis is to augment treatment approaches to ensure that liver histologies are only carried out when strictly necessary. The whole process is invasive for the patient, costly, and can be prone to substantial sampling variability. As such, proper validation with biomarkers is required to ensure that a liver histology is both necessary and the best approach to treating a patient with NASH or liver cirrhosis.
- FDA commits to increasing diversity in clinical trials
- How can biomarker trials be more patient-centric?
- Improving drug development timeframes for liver cirrhosis
There are some concrete general considerations to make in relation to surrogate marker qualification, with justifications for using surrogate endpoints as the primary focus in NASH identification and treatment. All research and progress towards identifying biomarkers in NASH should have some demonstrable impact, and new markers should help to predict a change in the clinical outcome.
In summary, there are a host of factors that biomarker readiness depends on. These include regulatory activity, such as its description in protocol and intended use. Additionally, its involvement in enrichment and surrogate endpoints, as well as confirmation. Some future needs of qualification include combination therapies and personal medicine, such as enrichment markers and outcome studies for cirrhosis.
Want to read more articles like this and take part in discussions on best-practice approaches to biomarkers in oncology? Check out our membership PLUS options. If you'd like to attend our upcoming Biomarkers US conference, visit our event website to download an agenda and register your interest.





.jpg)
