Selection Biomarkers: Shaping the Future of Drug Development and Regulatory Approval
By Ben Norris
When it comes to validating the efficacy of new drugs and therapeutics for future use, biomarkers are a surefire means of assessing a treatment’s method of action (MOA). Functionally speaking, selection biomarkers as validation methods ensure therapeutics are, like a good darts player, precise and accurate. This is increasingly key for securing regulatory approval, as Elizabeth Sheppard, Global Pricing & Market Access Director for Oncology Diagnostics at AstraZeneca, explained at Biomarkers 2023.
An Overview of Selection Biomarkers
In oncology, selection biomarkers are characteristics of certain conditions that may be measured in a patient’s tumour or bodily fluids. They can be used to identify patients who are most likely to benefit from a specific treatment while avoiding exposure to ineffective treatments. Selection biomarkers can be crucial to securing regulatory approval for a therapeutic in development by signposting its efficacy, or by pointing researchers towards alternative treatment approaches.
Implementing patient advocacy groups is one way to increase access to and improve communication between clinicians and patients.
“Almost eight in ten women diagnosed with breast cancer today are predicted to survive their disease for at least ten years... when you think about biomarker-targeted therapy, that's huge.”
Several kinds of selection biomarkers are used in oncology, including genetic biomarkers, protein biomarkers, and imaging biomarkers — biomarkers based on imaging techniques, such as MRI scans. Some examples of selection biomarkers in oncology include the HER2 protein in breast cancer, which is used to determine if a patient is a candidate for targeted therapy with drugs such as trastuzumab, and the EGFR mutation in lung cancer, which is used to predict responses to EGFR inhibitors.
Elizabeth Sheppard has worked in the oncological field of precision medicine for a significant portion of her career, and has seen improvements in treatment options for patients with breast cancer as they can be increasingly tailored to their specific needs without risking side effects. “Overall, almost eight in ten women diagnosed with breast cancer today are predicted to survive their disease for at least ten years or longer,” she said. “When you think about biomarker-targeted therapy and biomarker-targeted production, that’s huge.”
Targeted Therapies and Selection Biomarkers in Regulatory Approval
In the context of oncology agents, selection biomarkers have proven to be instrumental in increasing the likelihood of clinical trial success. As Sheppard explains, 75% of all new drugs for solid cancer approved by the FDA in 2021 were targeted therapies. Across the Atlantic, 86% of all new drugs for solid cancer approved by the EMA in 2020 were targeted therapies. While biomarker testing rates vary by region and type — uptake is highest for established biomarkers such as HER2 for breast cancer and BRAF for melanoma — the probability of trial success with selection biomarkers is much higher.
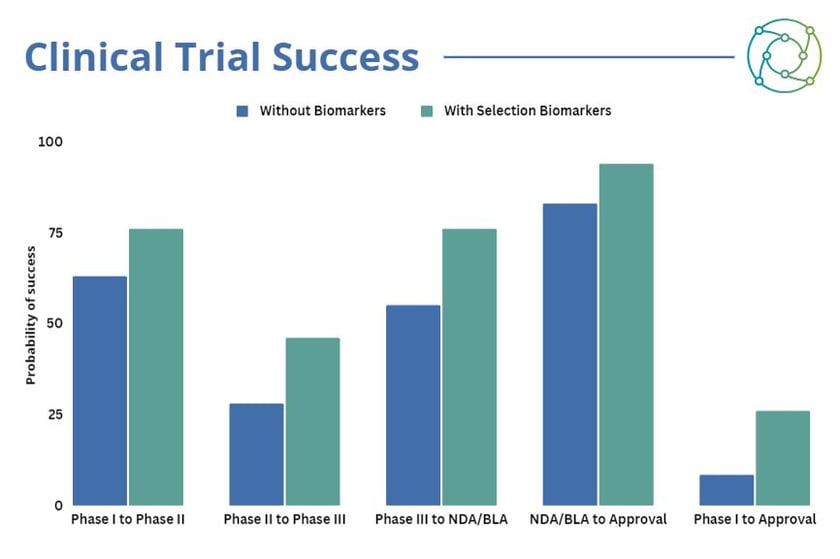
“If anyone knows how expensive clinical trials are, what does 17.5% actually mean?”, asked Sheppard. This is the difference in the likelihood of a new therapeutic progressing from Phase I to approval with a selection biomarker (25.9%) versus without a selection biomarker (8.4%). While the argument for including selection biomarkers in the validation methods for the development of a new therapeutic is concrete, integrating biomarker testing approaches can require some planning and consideration. “Getting the actual technology is key, because comprehensive next-gen sequencing is really expensive,” explained Sheppard. “Centralised laboratories are becoming more apparent because it’s a little easier to get all the rests to go to one place and it’s a little more economical.”
Current Barriers to the Broader Update of NGS in Oncology Clinical Practice
While the significance of selection biomarkers in achieving regulatory approval for cancer treatments is clear, there are a range of barriers that impede the broader uptake of next-gen sequencing (NGS) approaches in clinical practice to facilitate this. One of the biggest obstacles is the existing policy environment and infrastructure, concerning the implementation of NGS healthcare strategies: at present, there is a shortage of dedicated personnel and funding for NGS. Other issues include a lack of synchronisation between regulatory processes for treatment and diagnostics, as well as a limited awareness of the opportunities presented by NGS for healthcare policymakers.
Additionally, there are significant variations across Europe in terms of test access, multi-biomarker access and test quality, and at present there is a lack of standards to evaluate novel biomarkers and technologies. “Regulatory framework also plays an important part,” added Sheppard. These frameworks for biomarkers and precision medicine can vary by country, and are evolving significantly in both the EU and China. Greater consistency across national borders will help selection biomarkers to become more widespread as validation methods for precision medicine treatments.
Tomorrow’s Forecast for Selection Biomarkers in Precision Medicine
While constraints for the integration of NGS approaches in selection biomarker validation for new treatments will likely continue to be an issue in the coming years, the benefits are undeniable. “Prior to the advent of precision medicine, the treatment options for breast cancer were not tailored to the conditions of patients and had significant side effects,” said Sheppard. “It’s really helped us in finding the genetic information in the specific understanding of responses.”
- How can AI assist in biomarker discovery and validation?
- The legacy of the Coronavirus pandemic on international standards for new therapeutics
- New therapeutic targets discovered for aggressive breast cancer
Conventional approaches to cancer treatment have been superseded by more nuanced approaches, with a greater degree of variability for an individual patient’s condition enabled by selection biomarkers as validation methods. Testing for biomarkers can guide the use of precision medicines by selecting treatments that are most likely to work for a specific patient, rather than taking a more generalised approach to treatment provision and therapy.
Want to read more articles like this and take part in discussions on best-practice approaches to biomarkers in oncology? Check out our membership PLUS options to learn more. If you’d like to attend our upcoming Biomarkers US conference, visit our event website to download an agenda and register your interest.







.jpg)