Human Stem Cell Models of Neurodegeneration: Exploiting In Vitro Phenotypes to Support Drug Delivery
Presented by: Clare Jones, Chief Scientific Officer at Talisman Therapeutics
Transcribed by: Ben Norris
Human stem cell models offer invaluable insights into how neurodegenerative diseases such as Alzheimer’s and Parkinson’s affect the brain. For Clare Jones, Chief Scientific Officer at Talisman Therapeutics, the aim is to develop and apply the most translationally relevant in vitro models of neurodegeneration. Currently, this means working with induced pluripotent stem cell (iPSC)-derived neuronal and glial systems. The goal is to enable in vitro study of cellular dysfunction in fully human cell-based systems.
Studying Neurodegenerative Diseases Through Stem Cell Modelling
As Dr Clare Jones outlined at our Biomarkers UK event last November, human stem cell models form the basis of every project run at Talisman. “We do a lot of studies looking at the disease biology in affected cell types,” she told the audience in Manchester. “Neuronal dysfunction is a key factor in all neurological diseases, so it’s important to be able to study these cell types in vitro.” However, primary human neurons are not readily available for research. Before the introduction of iPSC-based systems, rodent models were applied to study neurodegeneration, but these were limited in their translational value. Jones explained that “human iPSC-derived neuronal models are really important for modelling and analysing neurodegenerative disease,” as they provide disease-relevant data to support drug discovery.
The process of in vitro differentiation mimics in vivo human neurogenesis, requiring a relatively long timeframe. Jones clarified that neurons are studied in vitro from day 60 to day 100, but these systems are scalable and well-characterised to ensure the neurons are "behaving like mature, functional neurons". Spontaneous network activity is present in cortical neurons in the laboratory around 60 days into the differentiation process, indicating they are synaptically connected. This network activity has been shown pharmacologically to be AMPA and N-methyl-D-aspartate (NMDA) receptor dependent. “A really important feature of neurons is that you want them to be electrically active,” added Jones. This ensures that the in vitro model behaves in the same way as a typical neuron.
“The power of these systems is that the neurons will show cellular dysfunction which is directly related to the mutation that they harbour.”
One of the strengths of the iPSC models are that they enable the investigation of neurons generated from disease-relevant backgrounds, such as Alzheimer’s. “The power of these systems is that the neurons, even at these relatively young stages in vitro – at around day 60 to day 100 – will show cellular dysfunction which is directly related to the mutation that they harbour.” Cellular dysfunction can be tracked through to the clinic, making these systems a powerful means of exploring early pathogenic events in Alzheimer’s disease.
Phenotypes Associated With Neurodegeneration: Tracking Amyloid β
Having established in vitro models of healthy neuronal networks, Jones moved on to discussing some of the phenotypes that are key indicators of neurodegeneration and Alzheimer’s disease. One such phenotype is Amyloid-β (Aβ) production, which has led to a significant volume of research on the amyloid cascade hypothesis of Alzheimer’s disease (Figure 1). Jones elaborated that “beta amyloid accumulation is widely considered to be fundamental to the progression and pathogenesis of Alzheimer’s disease.” The hypothesis holds that Aβ accumulation is key to disease progression: longer forms of Aβ, such as Aβ42, are believed to be the most toxic. Neurons harbouring these mutations exhibit variations in the production of different Aβ species (shown in Figure 1 as differences in Aβ40:Aβ42 or Aβ38:Aβ42 ratios). These altered ratios can be exploited experimentally to identify potential therapeutics that normalise Aβ production.
This Aβ phenotype is robust and very reproducible, as exemplified by a study aiming to identify new modulators of the pathway, as well as starting points for therapeutics. “To cut a long story very short, 1,200 compounds were screened using the Trisomy 21, or Down Syndrome model.” The results identified a class of compounds, avermectins, that increased relative production of short Aβ peptides, at the expense of longer, potentially more toxic peptides (Brownjohn et al., 2016, Stem Cell Reports). The study also demonstrated that these phenotypes are robust and suitable for screening for neurodegeneration.
High Content Imaging for Novel Biomarker Types in Neuroscience
The second cellular phenotype highlighted by Jones reflects endolysosomal dysfunction in Alzheimer's disease neurons, and can be visualised and quantified using high content imaging. Endosomal and lysosomal systems regulate sorting, recycling, and waste disposal processes of cellular proteins. Dysfunction of these systems has been highlighted as a key early event in the pathogenesis of multiple degenerative diseases in addition to Alzheimer's, including Parkinson’s and Huntingdon’s. Evidence from post-mortem samples supports this association, with a demonstrated abnormal accumulation of lysosomal dense bodies and autophagic vacuoles in dystrophic neurites (Nixon et al., 2005, J Neuropathol Exp Neurol).
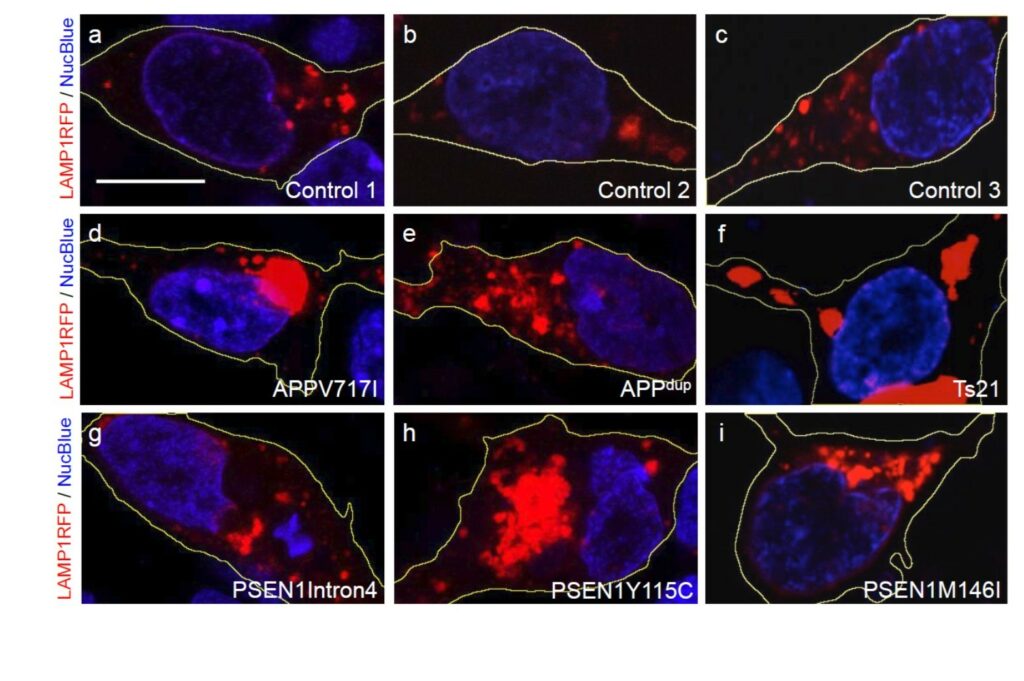
In human iPSC-derived neurons with APP and PSEN1 mutations, accumulation of lysosomes mirrors the observations from post-mortem Alzheimer’s disease brain, and can be quantified using high content imaging (Figure 2). Endolysosomal dysfunction can be analysed and quantified using immunofluorescence and high content imaging. These techniques can identify the accumulation of LAMP1+ lysosomes and p62+ autophagosomes in monogenic Alzheimer’s disease neurons. As Jones describes it, they ‘get stuck’ and cannot continue their maturation. These model systems are amenable to genetic manipulation and are therefore powerful tools for the mechanistic study of neurodegeneration.
- Pathway Mining and Proliferation Markers: Using Machine Learning to Enhance Genomic Analysis
- Watch: Precision Medicine in Autoimmune Disorders Webinar
- Using Spatial Technologies to Detect Biomarkers With Multiplex Immunofluorescence
In the case of PSEN1 mutations, it has been shown that endolysosomal phenotypes can be reversed by knocking out APP, providing insights into the mechanisms underlying these cellular deficits (Hung & Livesey, 2018). This system was also used to demonstrate proof of biology for APP reduction rescuing endolysosomal dysfunction in another monogenic Alzheimer’s model, in this case using antisense oligonucleotides (ASOs).
Applications for Future Neurodegenerative Disease Research
The Talisman team's work focuses on the preclinical phases of drug discovery and the developent and exploitation of human iPSC-derived neuronal and glial modelling systems. These are valuable and flexible experimental models that support neurological disease research, meaning that cellular dysfunctions in patients can be studied in cell-based systems in vitro. “These models are not modality specific,” Jones added. “Although the bulk of our work to date has been supporting small and large molecule projects, these model systems – being human – are well-suited for supporting gene therapy applications.” As this technology is further developed, human iPSC-derived systems will continue to replace animal-based models for the study of neurodegeneration.
One of the future challenges faced by the research team at Talisman is developing a model of myelinated axoms – axoms with a myelin sheath formed through cell-cell interactions with oligodendrocytes, another cell type found in the mammalian central nervous system (CNS). These more complex in vitro models, involving co-culture of multiple CNS cell types including astrocytes and microglia, offer great promise for developing more physiologically relevant assays and enable investigation of multiple disease relevant cell-cell interactions. Jones concluded that her research group's primary focus is building robust systems to address key questions around the study and treatment of diseases associated with neurodegeneration. “These stem cell models are really good now – they’re much better than they were ten years ago – but they’re still evolving, and the future is bright for further improving the relevance and predictive value of stem cell models to support drug discovery and combat neurodegenerative disease.”
Want to stay up to date with the latest Biomarker news? Register now for Oxford Global’s flagship event, Biomarkers US: In-Person. This is a must-attend forum covering the latest trends transforming biomarker and translational research





_1.jpg)