Bioassay Optimisation: How the Pandemic Reshaped International Standards for New Therapeutics

The race to develop a vaccine for Covid-19 saw new therapeutics trialled and validated at unprecedented speed. Bioassays — tests of a new therapeutic’s relative potency and biological activity — were crucial to the overall validation of the final product. Following the speed and success of the global vaccine rollout, the focus on this aspect of development has been on optimising bioassays for new modalities. While bioassays are powerful tests of relative potency and biological activity, care is required in their design to ensure they are a reliable indicator of potency.
But what does it mean to optimise a bioassay in the context of the drug development process? Should the focus be on constructing a procedure or process that is optimally adapted to the target in question, or is it more a question of conforming to regulatory requirements at the expense of pharmaceutical development speeds? A panel discussion at Biomarker Analysis & Tumour Microenvironment Europe in November 2022 set out to explore some potential answers based on industry insights and experience.
Bioassays: Past and Present
Across the spectrum of therapeutic modalities, every biological product from vaccines and cell therapies to complex therapeutic proteins — requires a bioassay to measure biological activity. As the broader therapeutic field has been propelled forward by scientific and technological advances, novel clinical applications have presented themselves. An imperative need to meet these swift developments with regulatory procedures for licensing and control that are necessarily more cautious has produced some debate concerning viable approaches and methods of best practice.
Putting the Covid-19 vaccine to one side for a moment, larger projects will often involve multiple research sites collecting and processing tissues in different ways. Alyson MacInnes, Director of Biomarker Discovery and Development at Byondis, speaks from her experience with the manufacture of antibody drug conjugates and monoclonal antibody therapies. “Being able to have a single protocol that consistently results in tissue where you are able to collect DNA is one of the key factors,” she said. “In terms of failure rates when you’re looking at sequencing, the majority of these failures are due to the way that the original sample is collected and processed.”
- FDA Set to Improve Subject Diversity in Clinical Trials
- Using Machine Learning to Predict Neurodegenerative Diseases
- Novel Immunotherapies for Bladder Cancer Treatment
In the context of bioassay optimisation for tumour research, certain obstacles are endemic to the laboratory. Keeping assay development to a single study site where researchers can enrol many patients and run the same assay repeatedly is “optimal, but also extremely difficult,” explained MacInnes. “Especially if it’s a rarer cancer.” Investigations run across multiple labs which may devote some of their resources to other studies can struggle with shipment delays and a dearth of materials, which can subsequently lead to sample degradation. Operating at multiple sites across regulatory borders can also pose issues if there are customs holdups.
GMP Development Considerations
If carefully planning where a bioassay will be used is important, so is accounting for the timing at which the assay will be deployed. A major consideration in pharmaceutical development speeds is the context of use: with potency bioassays used by the CMC (Chemistry, Manufacturing, and Controls), then ranges are typically different in terms of optimization. In this context, potency assays need to be introduced much earlier in the overall good manufacturing practice (GMP) timeline.
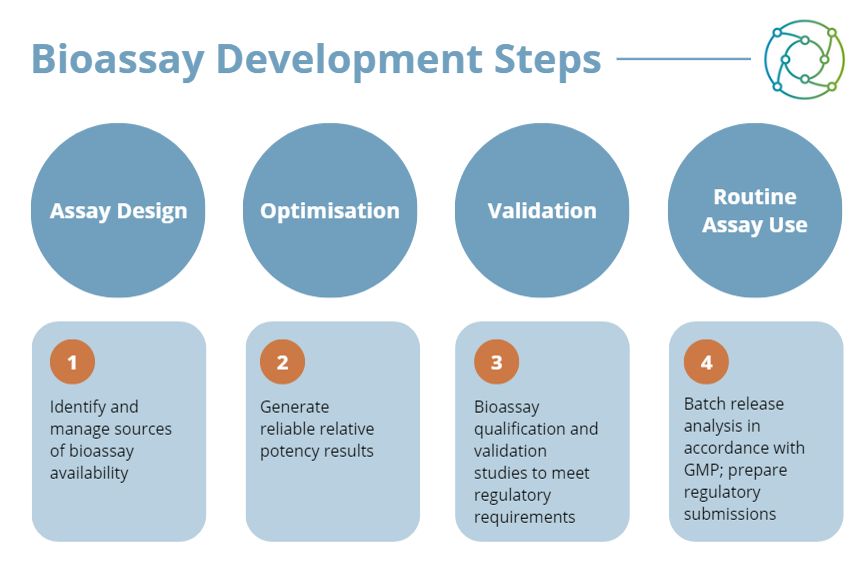
Another consideration to make in this context is that optimisation is completely different in terms of variability. One example is the work currently being done on primary cells tested with patient blood samples directly in the assay. With these, the variability becomes much higher, and researchers cannot focus on the same stringent cut-off for acceptance criteria. In this respect manufacturers of a new therapeutic or product have to be lenient, and modify their acceptance criteria to optimise accordingly.
How the Covid Vaccine set a Benchmark for Bioassay Validation
Coupled with advances in therapeutic and developmental approaches, there has been an imperative urgency to validate and approve new treatments. As animal models have declined in popularity for additional testing and validation, there are arguably less qualitative steps being carried out. “In a way it’s like we’re doing the bare minimum to get to regulatory standards,” said MacInnes.
This is not to say that corners are being cut or that the drug development process is any less rigorous as a result of increased pharmaceutical development speeds. It just means that in light of recent events such as the Coronavirus pandemic where the focus was on getting a vaccine to the public, there was a pressing demand to get assays up as quickly as possible. MacInnes asserted that while the pandemic was abating, the question was not ‘if’ another one happens, but ‘when.’
Figures in the industry have emphasised that the coronavirus vaccines were an exceptional case, but added they might represent a revolutionary approach to the quicker development of future therapies. Most vaccines need five to ten years of research and clinical development to be endorsed. However, the sheer amount of investment and resources that went into the development of the coronavirus vaccines meant this timeframe was greatly reduced. Further down the line, future cancer treatments may benefit from the same acceleration of the development pipeline.
Vaccine Production in Subsequent Pandemics
Following the successful global rollout of the Covid vaccine program, the next question is: are the big pharmaceutical companies in a position to ramp up the production of another developmental vaccine in the face of a subsequent pandemic? Daniel Larocque, Deputy Director in Innovation and Emerging Sciences at Sanofi, believes so, but thinks that the field for the drug development process is platform-driven at this time after the COVID crisis.
“There are opportunities to fast-track the validation process, even at a clinical level.”
The mRNA platform is being validated for other targets than just Covid, such as cancers, flu viruses, and cytomegalovirus (CMV). “There are opportunities to fast-track the process, even at a clinical level,” he said. “For example, running a phase I/II trial (efficacy endpoints and surrogates taken in account) instead of a classical phase I only trial (safety).” However, Larocque added that there were certain aspects — such as the phase III, which still needs time — that could not be easily fast tracked. Developments like this are tracked by the National Institute for Biological Standards and Control (NIBSC) in the UK.
Implications for Future Policy
Expanding on the extended applications of potency assays, Larocque queried the availability of international standards. “Do we think that these international standards — for example, NIBSC for the infectious diseases field — are available for immuno-therapeutics at this time?” In one panellist's view this was a difficult query to address, particularly regarding regulations on bioassays involved in the drug development process. “They are actually guidelines, to be honest,” he said. “A lot of assays — a lot of bioassays — have to be mechanism-of-action (MOA)-based. And in that area I think there’s still room for improvement.”
Regarding the pace of progress across the Atlantic, pipelines in EU trials tend to start earlier than in the US. The FDA moves a little more slowly than the EMA and displays a little more stringency in terms of pharmaceutical development speeds. However, by recognising data from initial trials in the EU, some of the cohort can be skipped on the US side. “The FDA accepted those things as standards, and that’s where we got some kind of harmonisation,” explained the panellist. However, demarcation for these particular MOA bioassays remains a grey area at present.
If you’d like to learn more about the latest industry trends concerning bioassay validation, check out the agenda for our flagship Biomarkers UK event. To see the full range of content across our Biomarkers Portal, view our membership options.







.jpg)