Universal Vaccines & Vaccine Therapeutics

In November 2022, Oxford Global’s Biologics series was delighted to host a panel of industry experts for the Discussion Group, Universal Vaccines & Vaccine Therapeutics. The group came together for one hour of conversation about the current landscape of approved immunotherapies and broadening its horizons.
Notable attendees included senior representatives from Akebia Therapeutics, Clover Biopharma, Merck, Pfizer, and Imperial College London to name a few.
What is Immunotherapy? What are Immunotherapeutic Vaccines?
Discussion Group leader Mark Doherty, Senior Medical Manager at GSK, opened the session with the following words: “When we talk about therapeutic vaccines, broadly speaking, we are referring to the prevention or treatment of disease with substances that stimulate the immune response.” However, Doherty explained how this is an “insufficient” definition: “what we really mean when we talk about therapeutics is treating existing disease.”
- Why We Develop Autoimmunity: Hyperstimulation of Genetically Prone Subjects
- 3-Dose Hepatitis B Vaccine Offers Full Protection for People with HIV
- Using Humanised Mice to Evaluate Vaccine Efficacy
He added, “and if we talk about immunotherapeutic vaccines, the approach we have is very different from the challenges facing prophylactic vaccines.” Prophylactic vaccines must stimulate a protective immune response — while immunotherapeutic vaccines must improve on the existing natural immune response. Any effective immune response generated must function alongside existing response, which “may require reversing or evading any existing immunosuppressive effect,” Doherty explained.
Existing Immunotherapeutic Vaccines
“If we look at existing and approved therapeutic vaccines, we have a total of only three,” Doherty continued. These are Bacillus Calmette-Guerin (BCG) for early-stage bladder cancer, Sipuleucel-T (Provenge) for metastatic castrate-resistant prostate cancer, and Talimogene Iaherparepvec (T-VEC or Imlygicis) for stage IIIb-IVM1c melanoma. Of these treatments, all of them are targeted for cancer as opposed to being a vaccine technology in itself, “which shows how the industry has evolved.”
Delving deeper, Doherty highlighted how only BCG can in actuality, be considered a classical vaccine. Provenge uses white blood cells, which are prepped in vitro and reinjected back into the patient. T-VEC or Imlygicis uses a similar approach, with GM-CSF being used to stimulate an immune response.
Understanding the Mechanism of Action for Bacillus Calmette-Guerin
Interestingly, BCG is a vaccine for childhood tuberculosis (TB). “So, what is it doing in cancer immunotherapy? And what is its mechanism of action?” Doherty asked. “The short answer is that we don’t really know.” BCG has a number of unexpected downstream benefits which were not predicted at the time in which the vaccine was first being used.
When the vaccine was first introduced in the 1920s in Sweden, there was a significant decline in mortality in vaccinated children (see figure 1). The 4% drop in overall mortality rates intrigued researchers working in the field because TB at the time was by no means responsible for 4% of all the deaths in Sweden. Only a tiny fraction of the apparent deaths prevented were due to TB, with most of the causes being non-TB or reduced neonatal deaths.
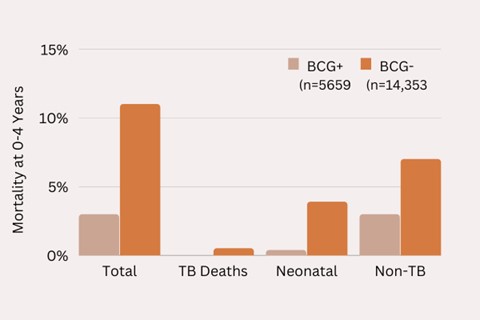
Figure. 1 – A graph showing the impact of BCG on all-cause mortality following the introduction for the BCG vaccination in Norrbotten Sweden, 1927 to 31.
Carl Naeslund, the researcher who published the results in 1932, wrote: “one could evidently be tempted to find an explanation for this much lower mortality among vaccinated children in the idea that BCG provokes a non-specific immunity.” In other words, the results showed a therapeutic effect unrelated to the initial disease. This was a phenomenon subsequently demonstrated across many studies and across many countries.
“Therapeutic vaccination has the potential to dramatically improve health in older adults but...more research to identify the mechanisms involved is urgently needed.”
Other notable findings linked the vaccine to different outcomes such as improved cognitive development, reduced risk of cancer among smokers, and improved glycemic control in type 1 diabetes patients, all of which – at first glance - appear to have nothing to do with each other. “When you dig a little deeper, the BCG vaccination has proven to generate a long-lasting immune response, which modulates and to some extent counters the negative effect that we see in patients with metabolic diseases,” Doherty said.
Broadening the Horizons for Immunotherapy
During the discussion part of the session, Doherty was asked about the future outlook for the industry. He stated that “therapeutic vaccination has the potential to dramatically improve health in older adults” but that “more research to identify the mechanisms involved is urgently needed.”
Following this, one audience member inquired about the data management strategies involved in identifying the therapeutic benefit of immunotherapy vaccines. Doherty responded by explaining how the rarity of the clinical research and trial will impact data handling. He pointed out that the small number of cases and the time these illnesses take to develop, mean that it is often hard to incorporate assessing vaccine impact on these into the data set-up of a standard clinical trial.
“As it stands, clinical intervention studies simply do not have the power to pick up on these downstream effects,” Doherty confirmed. “And at GSK we did try to look at whether it is possible to build these in as secondary endpoints, but most studies typically don’t have long enough follow up periods to facilitate this.” The hope moving forward is that the industry will soon discover an opportunity to better incorporate intervention for disease modification.
What’s Next in the Discussion Group Series?
To watch an on-demand recording of this Discussion Group, and to discover more about the exclusive offerings from our Biologics Membership Community, click here.
We will continue our Discussion Group series in the new year with sessions covering topics such as peptide and oligonucleotide chemistry, computational engineering, bispecific development, and many more. To stay tuned and gain exclusive access to the upcoming sessions in our Biologics series, click here.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.







